Clinical Oncology Research Journal
Research Article
A Preliminary Study on the Correlation between Il-6 And Il-17 Secretions in Human Systemic Diseases: Possible Existence of Two Different Origins of the Inflammatory Response
Lissoni P*, Rovelli F, Messina G, Borsotti GM, Frigerio S, Tosatto A, Aymerich T, Fede GD
Institute of Biological Medicine, Milan, Italy
*Corresponding author: Paolo Lissoni, Institute of Biological Medicine, Milan, Italy, Tel: +390258300445, E-mail: paolo.lissoni@gmx.com
Citation: Lissoni P, Rovelli F, Messina G, Borsotti GM, Frigerio S, et al. (2019) A Preliminary Study on the Correlation between Il-6 And Il-17 Secretions in Human Systemic Diseases: PossibleExistence of Two Different Origins of the Inflammatory Response. Clin Oncol Res J: CORJ-100004
Received date: 29 March, 2019; Accepted date: 29 May, 2019; Published date: 13 June, 2019
Abstract
The chronic inflammatory status, which characterizes human systemic diseases, including the neoplastic ad autoimmune pathologies, may depend on an enhanced activation of macrophage or TH17 lymphocyte systems, with a consequence increased production of IL-6 and IL-17, respectively.At present, however, it has to be established whether the inflammatory status may depend on a generalized activation of the immune system, or whether it may be primarily due to a preferential hyper function of the macrophage or the TH17 cell systems. This preliminary study was carried out to evaluate which interaction may exist between IL-17 and IL-6 secretions in patients with chronic inflammatory pathologies by analizing 40 consecutive patients, 22 of whom were suffering from metastatic cancer, and the other 18 patients were affected by autoimmune diseases. IL-6 and IL-17 were measured by an enzyme immunoassay on salivary samples collected during the morning. Abnormally high levels of IL-6 and IL-17 were observed in 13/40 (33%) and in 8/40 (20%) patients, respectively. Moreover, no significant correlation occurred between IL-6 and IL-17 concentrations. This preliminary study, by showing no correlation between IL-6 and IL-17 levels in human systemic diseases, seems to suggest the possibile existence of two different origins of the inflammatory response, consisting of macrophages system or TH17 lymphocytes, whose clinical physiopathological and prognostic significance has still to be established by longitudinal studies and monitoring changes in cytokine levels in relation to the clincal history of the immune alteration related-human systemic disease.
Keywords: Autoimmunity; Cancer; Inflammation; Interleukin-6; Interleukin-17
Introduction
Despite the in vitro activity of most cytokines is well known, the profile of cytokine secretion in the pathogenesis of the different human systemic diseases, has still to be better established from both pathogenetic and prognostic points of view. The controversial results reported in the literature concerning the blood concentrations of the different cytokines in the various systemic diseases could simply depend on the fact that the levels of each cytokine would represent the end-result of several interactions among the various cytokines, most of them are consisting of positive feed-back mechanisms, with a consequent multiple reciprocal stimulation, such as in the case of IL-6, namely produced by the macrophage system under stimulation of IL-1 beta, and IL-17 released from TH17 lymphocytes, as seen in Figure 1 . Then, each immune cell may be considered as a potential circulating micro-endocrine gland, which directly release into the blood its endocrine-like substances, represented by the same cytokines themselves. At present, it is known that the main inflammatory cytokines are consisting of IL-1beta and IL-6 secreted by macrophages [1], IL-17 produced by TH17 lymphocytes [2], and TNF-alpha, which may be produced by both macrophage and lymphocyte systems [3]. More in detail, Il-1 beta secreted by macrophage stimulates IL-6 from macrophage themselves [1], whereas IL-17 secretion is stimulated by TGF-beta in association with IL-6, IL-23 and IL-21 [4,5]. Moreover, it has been demonstrated that IL-17 stimulates the macrophage secretion of IL-1 beta, which in turn may promote the release of IL-17 [6]. Since both the macrophage cytokine IL-6 and the lymphocyte cytokine IL-17 may induce a chronic inflammatory status, it could be suggested the existence of two different potential origins of the inflammatory response, consisting of macrophage and T lymphocyte systems. However, because of the reciprocal stimulatory effect between IL-6 and IL-17, the two different possible sources of the inflammatory processes would allow a similar generalized inflammatory response. On the other side, the main anti-inflammatory cytokines are consisting of TGF-beta [7], which is namely produced by both regulatory T lymphocytes (T reg) and macrophages, and IL-10 [8], which may be secreted by M2 macrophage subset , TH2 cells and T reg lymphocytes. However, it has been remarked that both TGF-beta and IL-10 in addition to their anti-inflammatory activity may also exert a potent immunosuppressive effect on the antitumor immunity [7,8]. On the contrary, IL-2 [9], which is released from TH1 lymphocytes, and IL-12 [10], namely produced by dendritic cells, that represent the two main antitumor cytokines in humans, are able to play both inflammatory and anti-inflammatory effects. The possible anti-inflammatory activity of IL-2 is due to its potential stimulatory effect on TGF-beta secretion by T reg cells in the presence of high concentrations of TGF-beta itself [11,12], while the anti-inflammatory action of IL-12 is namely depending on its inhibitory effect on IL-17 secretion from TH17 cells [13]. On the other hand, their possible inflammatory actvity is due to an inhibitory action on T reg cell system [14], while that of IL-2 would be due to a generalized stimulation of both macrophage and lymphocyte systems for IL-2 [9]. Within the cytokine network, a fundamental important regulatory role is played by TGF-beta, which constitutes the most potent anti-inflammatory and immunosuppressive molecule in humans [7]. TGF-beta, namely produced by T reg lymphocytes and M2 macrophage subset [15], may block the inflammatory response by inhibiting the secretion of IL-2, IL-12 and IL-17, but on the same way it may stimulate IL-17 secretion in presence of IL-6, as well as IL-23 [2,4,5]. Therefore, the interactions between the two possible macrophage or TH17 lymphocyte sources of the inflammatory status would be mainly regulated by TGF-beta itself. Finally, IL-18 would also play an important role in amplifying the inflammatory processes [16]. Both neoplastic and autoimmune diseases are characterized by an excessive chronic inflammatory status, whose primary origin, however, could be different. According to the experimental data available up to now, autoimmunity-related inflammation would be mainly induced by IL-17 released by TH17 lymphocytes [16], with a consequent decline in TGF-beta levels because of the inibitory effect of IL-17 on T reg cells, which represent the main source for TGF-beta. On the contrary, cancer-related chronic inflammatory status would mainly depend on an enhanced macrophage system activation [17], which promotes the stimulation of T reg cell system, with a following increase in TGF-beta secretion. Therefore, the main pathogenetic cytokine difference between neoplastic and autoimmune diseases , which are both characterized by an increase in inflammatory cytokine concentrations, namely IL-6, IL-1 beta and TNF-alpha, would consist of TGF-beta blood concentrations, which tend to be abnormally enhanced in the metastatic cancer [7,12] and low during the exacerbation phases of the various autoimmune diseases [18]. The difference between cancer and autoimmunity would also regard the prognostic significance of TGF-beta concentrations, whose increase has appeared to predict a better prognosis in the autoimmune diseases [18], and a poor prognosis in the neoplastic ones [12]. The in vivo concentrations of the various cytokines may be determined either in the blood, or at salivary level, and a good correlation has been observed between blood and salivary cytokine concentrations [19]. The present preliminary study was performed in an attempt to evaluate which correlation may exist between the concentrations of IL-17A, that constitutes the main isoform of IL-17 family, and those of IL-6, which respectively reflect a macrophage or a TH17 lymphocyte origin of the inflammatory process, in a group of patients suffering from cancer or autoimmune diseases.
Materials and Methods
The study included 40 consecutive patients (M/F: 15/25; median age 48, range 23-76), who were affected by advanced solid tumors (n=22), or by autoimmune pathologies (n=18). All cancer patients showed a metastatic disease, while patients with autoimmune diseases were investigated during the remission phase of their pathology. Tumor histotypes were, as follows: breast cancer: 9; nonsmall cell lung cancer: 7; colorectal cancer: 6. The autoimmune diseases were consisting of multiple sclerosis (n=4), psoriasis (n=4), rheumatoid arthritis (n=3), systemic lupus erythematosus (n=3), sclerodermia (n=2), thyroiditis (n=2). For cytokine measurement, salivary samples were collected at 8.00 A.M after an overnight fast. Salivary levels of IL-6 and IL-17A were detected by an enzyme immunoassay and commercially available kits. TNF-alpha salivary levels were also detected in the same samples by an enzyme immunoassay and commercial kits. Normal levels of IL-6, IL-17 A, and TNF-alpha observed in our laboratory (95% confidence limits) were less than 51pg/ml for IL-174, less than 6.5pg/ml for IL-6, and less than 5.5pg/ml for TNF-alpha. Data were reported as mean +/- SE, and statistically analyzed by the Student’s t test, the chi-square test, and the coefficient of correlation, as appopriate.
Results
Abnormally high levels of IL-6, IL-17A, and TNF-alpha were observed in 13/40 (33%), in 8/40 (20%), and in 15/50 (38%) patients, respectively, without, however, no significant difference between cancer patients and those with autoimmune diseases ( IL-17A: 4/22 (18%) vs 4/18 (22%); IL-6: 6/22 (27%) vs 7/18 (39%); TNF-alpha (9/22 (41%) vs 6/18 (33%). Simultaneous high levels of both IL-6 and IL-17 A occurred only in 3/40 (8%) patients. IL-17A mean salivary levels in relation to those of IL-6 and TNF-alpha are reported in Table 1, while Table 2 shows IL-6 concentrations in relation to those of IL-17A and TNF-alpha. Patients with normal levels of IL-17A showed higher mean levels of IL-6 than those with high IL-17A concentrations, even though the difference was not statistically significant. IL-17A mean levels were higher in patients with normal IL-6 values than in those with high IL-6 concentrations, but also this difference was not significant. Finally, both patients with high IL-17A or IL-6 values showed higher levels of TNF-alpha than those with normal IL-17A or IL-6 concentrations, but none of these differences was statistically significant. Finally, no significant correlation occurred between IL-6 and IL-17A concentrations (Pearsonr= -0.2, P 0,05).
Discussion
The results of this preliminary study, by excluding the existence of a positive correlation between IL-6 and IL-17 endogenous levels seems to confirm the possible existence of at least different origin of the inflammatory response in patients suffering from a chronic inflammatory status due to a systemic disease, including advanced cancer and autoimmune pathologies. However, the too low number of patients does allow us to identify possible differences in the profile of cytokine secretion neither between cancer patients and those with autoimmune diseases, nor in relation to the different tumor histotypes, as well as to the type of autoimmune pathology. The regulation of the interactions between IL-17 and IL-6 would be mainly due to the concentrations of TGF-beta itself, since TGF-beta alone may inhibit IL-17 production, which in contrast is stimulated by TGF-beta in association with high concentrations of IL-6, as well as those of IL-21 and IL-23 [4,5,7]. IL-18, which is mainly produced by both endothelial and epithelial cells, would have also to be taken into consideration in the modulation of IL-6 – IL-17 interactions, since it has been shown to inhibit IL-17 secretion and to promote T reg cell differentiation [20]. Then, because of the reciprocal stimulatory effect between IL-18 and IL-1 beta, which is released from macrophages, IL-18 would mainly amplify macrophage-mediated inflammatory response rather than that induced by TH17 lymphocytes. Therefore, further studies, by concomitantly evaluating TGF-beta and IL-18 concentrations, would be required to confirm that IL-18 may induce a preferential macrophage-dependent inflammatory response. In any case, the importance of IL-18 in the induction of the inflammation is also confirmed by the evidence of high IL-18 levels in main chronic systemic inflammatory diseases [21]. On the contrary, IL-22 would play a major anti-inflammatory action [22]. Moreover, according to the knowledgements available up to now [20], IL-18 would be involved in the pathogenesis of allergy, since the diminished secretion of gamma-interferon (IFN), that constitutes one of the main allergy-associated cytokine anomalies [23], would be due to an enhanced production of IL-18 itself. An enhanced production of IL-25, which stimulates TH2 differentiation, has also been proven to be involved in the allergy [20,23]. Then, even though the clinical reality is more complex, at present itis possible to affirm that autoimmunity is mainly characterized by an enhanced IL-17 secretion in assocation with low levels of TGF-beta and IL-10. On the contrary the neoplastic diseases are characterized by high levels of TGF-beta and IL-10 in association with low levels of IL-2 and IL-12 in the advanced disease, while there are still controversial data concerning IL-17 secretion. Finally, allergy would be characterized by low levels of gamma-IFN in association with high IL-18 concentrations. However, since the profile of cytokine secretions may change during the clinical course of the disease either in cancer patients, or in those with autoimmune disease in relation to the exacerbation or remission of the same disease, further longitudinal clinical studies in a greater number of patients, by monitoring changes in cytokine levels in relation to the clinical history of their pathology, will be required to better understand the role of the single inflammatory cytokine in influencing the mechanims involved in determining the onset and the maintenance of the chronic inflammatory response in both neoplastic and autoimmune diseases. In conclusion, this preliminary clinical investigation, by excluding the evidende of a positive correlation between IL-6 and IL-17 endogenous concentrations, which respectively reflect the activation of the macrophage or the TH17 lymphocyte systems, would suggest the possible existence of two different cytokine origins of the inflammatory status, consisting of TH17 cells and macrophages, which may be clinically identified by the evidence of abnormally high levels of IL-17 or IL-6, respectively. From a phylogenetic point of view, macrophage-induced inflammatory status preceedes that generated by the lymphocyte system. Moreover, according to the data available up to now,from a schematic point of view,it is possible to suggest that macrophage-dependent inflammatory status, which is characterized by high levels of both IL-1 beta and IL-6, would be mainly involved in the pathogenesis of advanced cancer-related chronic inflammatory status, whereas TH17 lymphocyte-related inflammatory response, as shown by the evidence of abnormally high IL-17 levels, would be mainly responsible for the induction of the inflammatory status occurring in the autoimmune disorders.
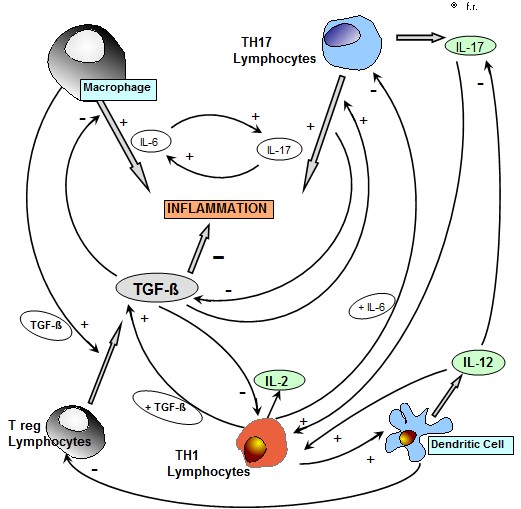
Figure 1: Interaction between the main inflammatory cytokines , IL-6 , IL-17 and TNF-alpha in patients with chronic inflammatory pathologies ( metastatic cancer and autoimmune diseases).
|
IL-17Apg/ml |
n |
IL-6 pg/ml |
TNF-alpha pg/ml |
|
Normal IL-17 values High IL-17 values |
32 8 |
4.8 +/- 0.4 3.4 +/- 0.5 |
4.2 +/- 03 5.8 +/- 06 |
Table 1: Salivary levels (mean +/- SE) of IL-17A in relation to IL-6 and TNF-alpha values in patients with systemic diseases.
|
IL-6 pg/ml |
n |
IL-17A pg/ml |
TNF-alpha pg/ml |
|
Normal IL-6 values High IL-6 levels |
27 13 |
37 +/- 5 26 +/- 4 |
4.6 +/- 0.3 5.1 +/- 0.4 |
Table 2: Salivary levels (mean +/- SE) of IL-6 in relation to IL-17A and TNF-alpha values in patients with systemic diseases.
Citation: Lissoni P, Rovelli F, Messina G, Borsotti GM, Frigerio S, et al. (2019) A Preliminary Study on the Correlation between Il-6 And Il-17 Secretions in Human Systemic Diseases: PossibleExistence of Two Different Origins of the Inflammatory Response. Clin Oncol Res J: CORJ-100004