Annals of Medical & Surgical Case Reports
Research Article
Nitroglycerin in Suboptimal Versus the Remaining Doses in Hypertensive Crises Regards Propylene Glycol; Efficacy and Safety (Nitroglycerin on Trace Study); Retrospective Observational Study
Elsayed YMH*
Critical Care Unit, Fraskour Central Hospital, Damietta Health Affairs, Egyptian Ministry of Health (MOH), Damietta, Egypt
*Corresponding author: Yasser Mohammed Hassanain Elsayed, Critical Care Unit, Fraskour Central Hospital, Damietta Health Affairs, Egyptian Ministry of Health (MOH), Damietta, Egypt, Tel: +201141292365; Email: dryaser24@yahoo.com
Citation: Elsayed YMH (2019) Nitroglycerin in Suboptimal Versus the Remaining Doses in Hypertensive Crises Regards Propylene Glycol; Efficacy and Safety (Nitroglycerin On Trace Study); Retrospective Observational Study. Ann Med & Surg Case Rep: AMSCR-1000021
Received date: 16 September, 2019; Accepted date: 26 September 2019; Published date: November 15, 2019
Abstract
Background: Hypertensive crises represent the most immediate danger to those afflicted and the most dramatic proof of the lifesaving potential of antihypertensive therapy. It is one of the most serious presentations in the emergency department for hypertensive patients. Hypertensive crises are largely preventable and often result from inadequate management of hypertension or poor adherence to therapy.
Method of study and patients: My study was an observational retrospective for 1043 cases. The study was conducted in both Ras El-Bar Central Hospital and Fraskour Central Hospital; in the Intensive Care Units. The author reported the 1043 cases of hypertensive crises over nearly 48-months, started from July 10, 2015, and, ended on Jul 10, 2019. Different doses within the normal range of i.v nitroglycerin was given. Both adverse effects (safety) and anti-hypertensive responses (efficacy) were recorded.
Results: Range of age in group I was 24-90 years and in group II was 24-88 years but with insignificant P-value (0.472). There is predominant female sex but with insignificant P-value (0.286). Predominant side effects in group II vs. group I with P-value (<0.001) was significant in group I vs. group II. There is no significant difference in response for both groups after nitroglycerine i.v infusion with P-value (0.735).
Conclusions: The author concluded that nitroglycerine is effective and safe in the treatment of hypertensive emergencies with the lowest vs. the remaining other nitroglycerin infusion doses. Regards the drug efficacy, no significant difference between the lowest and remaining other nitroglycerin infusion doses in treating hypertensive crises. Using the lowest nitroglycerin infusion dose in treating hypertensive crises safer, economically saver, and less anxious regards the serious adverse effects. Propylene glycol may be the suggested inducing agent for the side effects and its severity with increasing nitroglycerin infusion doses.
Keywords: Efficacy and safety; Hypertension crises; Lower versus remaining doses; Propylene glycol; Nitroglycerin on trace study; Nitroglycerin
Abbreviations
ACS : Acute coronary syndromes
AMI : Acute myocardial infarction
BP : Blood pressure
cGMP : cyclic GMP
DBP : Diastolic blood pressure
ECG : Electrocardiogram
ESH : European Society of Hypertension
ESO : European Stroke Organization
Euro-STAT : European registry for studying the Treatment of Acute hypertension
EUSI : European Stroke Initiative
FMD : Flow-mediated dilation
IUPAC : International Union of Pure and Applied Chemistry
LV : Left ventricular
LOAEL : The lowest-observed-adverse-effect level
MEG : Methyl ethyl glycol
NOAEL : The no-observed-adverse-effect-level
NO : Nitrous oxide
PCWP : Pulmonary capillary wedge pressure
PG : Propylene glycol
SBP : Systolic blood pressure
USA : United States of America
VMAC trial : Vasodilatation in the Management of Acute Congestive Heart Failure trial
Introduction
Hypertensive crises
Importance: Hypertension is an extremely common clinical problem. Approximately 1% of these patients will develop hypertensive crises at some point in their lifetime [1]. Hypertensive crises represent the most immediate danger to those afflicted and the most dramatic proof of the lifesaving potential of antihypertensive therapy [2]. It is one of the most serious presentations in the emergency department for hypertensive patients. Hypertensive crises are largely preventable and often result from inadequate management of hypertension or poor adherence to therapy [3]. Patients with hypertensive crises may require an immediate reduction in elevated blood pressure to prevent and arrest progressive end-organ damage [1]. A hypertensive crisis is a present when markedly elevated blood pressure is accompanied by progressive or impending acute target organ damage or death [3,4]. Prompt recognition, based primarily on physical signs and symptoms is essential. Appropriately aggressive therapy will often result in a satisfactory outcome [4,5]. Antihypertensive drugs that will be lowered aggressively of markedly elevated blood pressure within minutes to hours has changed the concepts of definition and therapy of hypertensive emergencies and urgencies [6,7]. Prompt treatment of hypertensive emergencies is only required to prevent target organ damage and death [8,9]. Blood pressure control in a hypertensive emergency should be attained as expeditiously as possible with a short-acting titratable intravenous antihypertensive medication to prevent ongoing and potentially permanent end-organ damage [10,11]. The admission to an intensive care unit with a closely supervised inpatient setting for an immediate BP reduction with titratable intravenous antihypertensive drugs is mandatory to arrest progressive organ damage. Therapeutic protocols and target BP in the single patient should be based on the clinical presentation and a prompt diagnostic workup [11,5]. Rapid triage is necessary to differentiate those who can safely be sent home and those need hospitalization for more serious problems [12]. On the other hand, overzealous or uncontrolled reduction in blood pressure may result in coma, stroke, myocardial infarction, acute renal failure, or death [13].
Epidemiology: Hypertensive crises is a common issue in the emergency department [14]. Worldwide, hypertension may affect as many as 1 billion people and be responsible for approximately 7.1 million deaths per year. It is estimated that approximately 1% of patients with hypertension will, at some point, develop a hypertensive crisis [15].
Definition and Classification: An acute elevations in blood pressure that are associated with end-organ damage are called hypertensive crises [1]. Hypertensive crises with blood pressure > 180/120 mmHg are common issues in the emergency department [14]. If sudden elevation in systolic (SBP) and/or diastolic blood pressure (DBP) that are associated with acute end-organ damage (cardiovascular, cerebrovascular, or renal) is defined as a hypertensive crisis or emergency. In contrast, acute elevation in SBP and/or DBP not associated with evidence of end-organ damage is defined as a hypertensive urgency [8]. The severity of hypertensive crises is determined by the presence of target organ damage rather than the level of blood pressure [16].
Presentations and Differentiation: Differentiation between hypertensive emergencies and urgencies is related more to the presence of acute organ involvement than to BP elevation, per se. Hypertensive urgencies can be treated outside the intensive care unit with oral antihypertensive medications for 24-48 hours [11]. Hypertensive urgencies is including: severe uncomplicated essential hypertension, severe uncomplicated secondary hypertension, postoperative hypertension, hypertension associated with severe epistaxis, drug-induced hypertension, rebound hypertension, hypertensive encephalopathy, cerebral infarction, cerebral hemorrhage, advanced retinopathy, acute coronary syndromes, acute heart failure, aortic dissection, acute renal failure, and an eclampsia but hypertensive emergency is including: hypertensive encephalopathy, hypertension associated with acute cerebrovascular disease, hypertension associated with pulmonary edema, hypertension associated with acute coronary syndromes, hypertension associated with dissecting aortic aneurysm, pheochromocytoma, hypertension associated with acute renal failure, an eclampsia, micro-angiopathic anemia and severe hypertensive crises related to anxiety, panic attacks, or pain [11].
Target for Blood Pressure: Current guidelines suggest pharmacological intervention if systolic BP exceeds 180 mm Hg [17]. There is strong evidence to support treating hypertensive persons aged 60 years or older to a BP goal of less than 150/90 mmHg and hypertensive persons 30 through 59 years of age to a diastolic goal of less than 90 mmHg; however, there is insufficient evidence in hypertensive [1]. Blood pressure treatment in the acute stage of stroke: The ESO recommends a reduction to
Protocol: The best clinical setting in which to achieve this blood pressure control is in the intensive care unit, with the use of titratable intravenous hypotensive agents [1,3,15]. Complete evaluations in patients who present with a hypertensive crisis to effectively reverse, intervene, and correct the underlying trigger, as well as improve long-term outcomes after the episode [5]. The blood pressure must be quickly lowered to a target range of at least
Nitroglycerin
Pharmacology: Nitroglycerin is a potent venodilator and only at high doses affects arterial tone [15]. Once nitroglycerin is converted to nitric oxide, it activates guanylate cyclase and stimulates the production of cyclic GMP (cGMP). This produces smooth muscle relaxation, mainly in the venous system, and reduces myocardial preload [21]. Nitroglycerin reduces BP by reducing preload and cardiac output; this increases the severity of the hyperadrenergic state characteristic of acute postoperative hypertension [22]. Since a hypertensive crisis is usually accompanied by left ventricular failure, pulmonary edema, angina pectoris, or infarction, nitroglycerin has been definitively shown positively to influence these conditions, and preference should be given to nitroglycerin in the treatment of hypertensive crises [23]. Nitroglycerin may be advantageous in patients with significant coronary artery disease and the preferred in acute coronary syndrome [24,25] with or without hypertension, acute heart failure [25], cocaine toxicity/pheochromocytoma [26], perioperative hypertension [27], volume overload, and pulmonary edema [28]. Endothelium-independent dilator response can be tested by low-dose sublingual n nitroglycerin [29]. Sodium nitroprusside and nitroglycerin are usually used to assess endothelium-independent vasodilation [30].
Administration: Intravenous nitroglycerin, however, is usually a better method to administer nitroglycerin because the dose can be rapidly adjusted upward or downward depending on the clinical and hemodynamic response [31].
For the treatment of hypertension, the initial dose of nitroglycerin is 5 µg/min by i.v infusion. The dose may be increased in increments of 5 µg/min every 3-5 minutes to a maximum rate of 20 µg/min. If the BP response is inadequate at 20 µg/min, the dose may be increased by 10 µg/min every 3-5 minutes, up to a maximum rate of 200 µg/min. When a partial response is achieved, dosing increments are made more carefully. Drug onset is within 2-5 minutes, and the duration of action is 5-10 minutes, with a half-life of 1-3 minutes [21]. Nitroglycerin should be infused at an initial rate of 5 mcg/min (or even 2.5 mcg/min in patients with borderline hypotension with eventual rates of 1000 mcg/min in some patients. There are risks of tolerance induction and subsequent rebound. Given that even 10 mcg/min nitroglycerin induces some degree of tolerance within 24 hours, [32] a maximal infusion rate of 16 mcg/min is recommended in most cases [33].
Presentations and nitroglycerin: Intravenous nitroglycerin is often chosen for patients with myocardial ischemia since it dilates coronary vessels and decreases myocardial wall tension and oxygen consumption [34]. Myocardial ischemia or myocardial infarction may be associated with hypertension, which usually results from a preexisting high BP exacerbated by pain and agitation. In this setting, intravenous nitrates are useful in reducing systemic vascular resistance, as well as left ventricular preload, and in improving coronary perfusion [35]. Administration of low-dose nitroglycerin (≈ 60 µg/min) as an adjunct to other i.v antihypertensive therapy may be beneficial for patients with hypertensive emergencies associated with acute coronary syndromes or acute pulmonary edema [15,21]. Aortic dissection is the most dramatic and rapidly fatal complication associated with hypertensive emergencies. Acute BP reduction is essential to reduce shear forces on the damaged aorta. Treatment aims is to decrease systolic BP as rapidly as possible down to 100-110 mmHg and simultaneously control tachycardia resulting from the sympathetic activation [35].
The acute cardiogenic pulmonary edema necessitates rapid and specific interventions, including ventilation and reduction of left ventricular preload and afterload. The first-line treatment of this condition is based on intravenous administration of nitrates and loop diuretics. If this approach is not effective, vasodilators, such as urapidil, nicardipine, or sodium nitroprusside, are also indicated [11]. In acute pulmonary edema from various causes, including AMI, nitroglycerin can be strikingly effective, with some risk of precipitous falls in BP and of tachycardia or bradycardia. Nitroglycerin can relieve dyspnea within 15 to 20 minutes, with a fall of LV filling pressure and a rise in cardiac output [36]. Administration of low-dose nitroglycerin (≈ 60 µg/min) as an adjunct to other i.v. antihypertensive therapy may be beneficial for patients with hypertensive emergencies associated with acute coronary syndromes or acute pulmonary edema [20].
It is essential to control arterial blood pressure (BP) in both hemorrhagic and ischemic stroke patients to decrease morbidity following an acute event and decrease the long-term risk of stroke recurrence [37]. BP elevations commonly accompany ischemic stroke in previously hypertensive and in normotensive subjects. Stroke-related hypertension has been hypothesized to result from lesions in cerebral areas causing an impaired neurogenic control of the cardiovascular system [11]. Nitroglycerin is one of the commonly used drugs for the management of postoperative surgical hypertension [38]. But nitroprusside is often considered a drug of choice in hypertensive emergencies these settings [39]. Local application of vasodilators, such as nitroglycerine, may be useful to prevent or revert spasms of renal arteries, and the energy delivered is lower than that used for cardiac electrophysiological procedures [40].
Adverse effect: The main side effects are headache and hypotension, both of which respond to decrease or cessation of the infusion [41]. Severe hypotension and reflex tachycardia have been reported in volume-depleted patients within minutes of initiating nitroglycerin infusion [22]. Headache is the most common adverse effect, and methemoglobinemia is a rare complication of prolonged nitroglycerin therapy [21]. Methemoglobin is formed during the administration of all organic nitrates, but its mean concentration in patients receiving nitroglycerin for 48 hours or longer averaged only 1.5%, with no clinical symptoms [42]. Rebound is the abrupt increase in anginal frequency during accidental nitrate withdrawal (e.g., displacement of an intravenous infusion) or during nitrate-free periods [43]. The underlying mechanisms of a rebound are unopposed vasoconstriction (angiotensin II, catecholamines, and endothelin) during nitrate withdrawal with attenuation of net vasodilator effect of NO [44]. Tolerance to the hemodynamic effects of nitroglycerin may limit its clinical usefulness [21].
Contraindication: Nitrates should not be administered to patients with a systolic BP of less than 90 mm Hg, patients with right ventricular infarction, or those who received sildenafil (or it's equivalent) in the last 24 hours [45].
Comparison nitroglycerin with sodium nitroprusside and nifedipine: Although sodium nitroprusside is a rapid-acting and potent antihypertensive agents, it may be associated with significant toxicity [1]. Sodium nitroprusside is an extremely toxic drug and its use in the treatment of hypertensive emergencies should be avoided [15]. Furthermore, the short-acting calcium channel blocker nifedipine is associated with significant morbidity and should be avoided [9].
Propylene Glycol
Structure and properties: Propylene glycol, also called propane-1,2-diol, is a synthetic organic compound with the chemical formula C3H8O2 [46]. The excipient of nitroglycerin solutions is propylene glycol. High concentration solutions of nitroglycerin contain propylene glycol. Intravenous administration of an excipient has been seen in some people, particularly with large dosages [47].
Propylene glycol toxicity: Propylene glycol toxicity may include: hypotension, bradycardia, QRS and T-wave abnormalities on the ECG, arrhythmia, cardiac arrest, serum hyper osmolality, lactic acidosis, and hemolysis [48]. Serious toxicity generally occurs at plasma concentrations over 4 g/L, which requires extremely high intake over a relatively short period, or when used as a vehicle for drugs or vitamins given intravenously or orally [49].
Symptomatology is dose-dependent, ranging from drowsiness to stupor, deep unconsciousness, and coma. Other signs include hyper osmolality of serum, lactic acidosis, and hypoglycemia [50,51]. Rapid intravenous injection of preparations of drugs containing propylene glycol as a solvent (in significant amounts) may cause unconsciousness, arrhythmias and even cardiac arrest [50,51]. PG is not acutely toxic to aquatic organisms except at very high concentrations. Repeated exposures of rats to propylene glycol (PG) did not result in side effects at levels up to 10% in water (estimated at about 10 g/kg bw/day) or 5% in feed (dosage reported as 2.5 g/kg bw/day) for periods up to 2 years. However, in cats, two studies of at least 90 days’ duration show that a species-specific effect of increased Heinz bodies was observed (NOAEL=80 mg/kg bw/day; LOAEL=443 mg/kg bw/day), with other hematological effects (decrease in number of erythrocytes and erythrocyte survival) reported at higher doses (6-12% in diet, or 3.7-10.1 g/cat/day) [51].
Method of study and patients
My study was an observational retrospective for 1043 cases. The study was conducted in both Ras El-Bar Central Hospital and Fraskour Central Hospital; in the Intensive Care Units. The author reported the 1043 cases of hypertensive crises over nearly 48-months, started from July 10, 2015, and, ended on Jul 10, 2019. (Table 1) Different doses within the normal range of i.v nitroglycerin was given. Both adverse effects (safety) and anti-hypertensive responses (efficacy) were recorded. The cases were selected in the Emergency Department then admitted to the Critical Care Unit.
The cases were divided into two groups:
Eligibility criteria
Exclusion criteria
The cases were selected depending on two criteria
Each patient underwent to rapid complete history, clinical examination, and some workup
Age: The range of age in group I was 24-90 and in group II was 24-88 (Figure 1) but with insignificant P-value (0.472) (Table 1).
Sex: Predominant female sex was cleared (Table 2). The percent of sex in group I for female vs. male was 55.8% vs.44.2% (No. 364 vs. 288) and in group II was 52.4% vs.47.26% (No. 205 vs. 186) (Figure 2) but with insignificant P-value (0.286) (Table 2).
Hypertension-associated conditions: P-value (<0.001) was significant in comparing group I and group II (Table 3). With predominant hypertension-associated conditions in group II vs. group I (Table 3) and (Figure 3).
Side effects: P-value (<0.001) was significant in comparing group I and group II (Table 4). With predominant side effects in group II vs. group I (Table 4 and Figure 4). This is indicating that the increase in nitroglycerine i.v. infusion dose is directly related to increasing to the side effects.
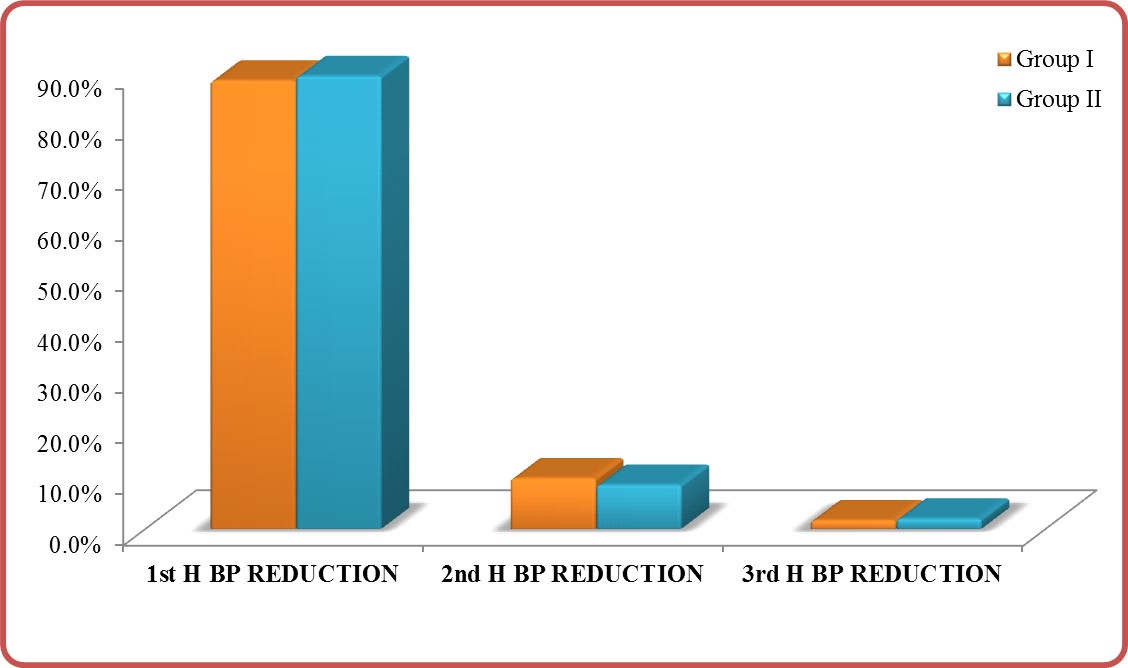
Response (Efficacy): P-value (0.735) was insignificant in comparing group I and group II (Table 5) in the first three hours’ post-nitroglycerine i.v. infusion (Table 5) and (Figure 5). This is indicating that there is no significant difference in response for both group after nitroglycerine i.v. infusion.
Conclusion
The author concluded that nitroglycerine is effective and safe in the treatment of hypertensive emergencies with the lowest vs. the remaining other nitroglycerin infusion doses. Regards the drug efficacy, no significant difference between the lowest and remaining other nitroglycerin infusion doses in treating hypertensive crises. Using the lowest nitroglycerin infusion dose in treating hypertensive crises safer, economically saver, and less anxious regards the serious adverse effects. Propylene glycol may be the suggested inducing agent for the side effects and its severity with increasing nitroglycerin infusion doses.
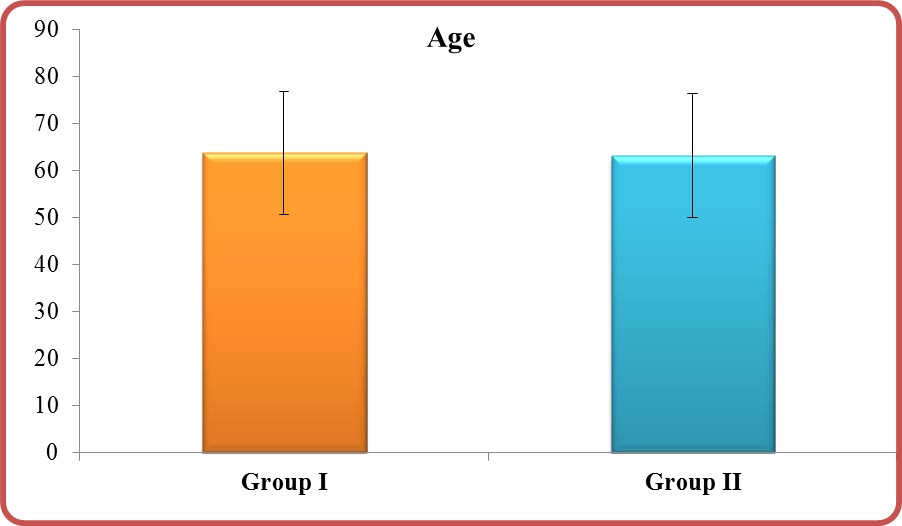
Figure: Age in group I vs. group II.
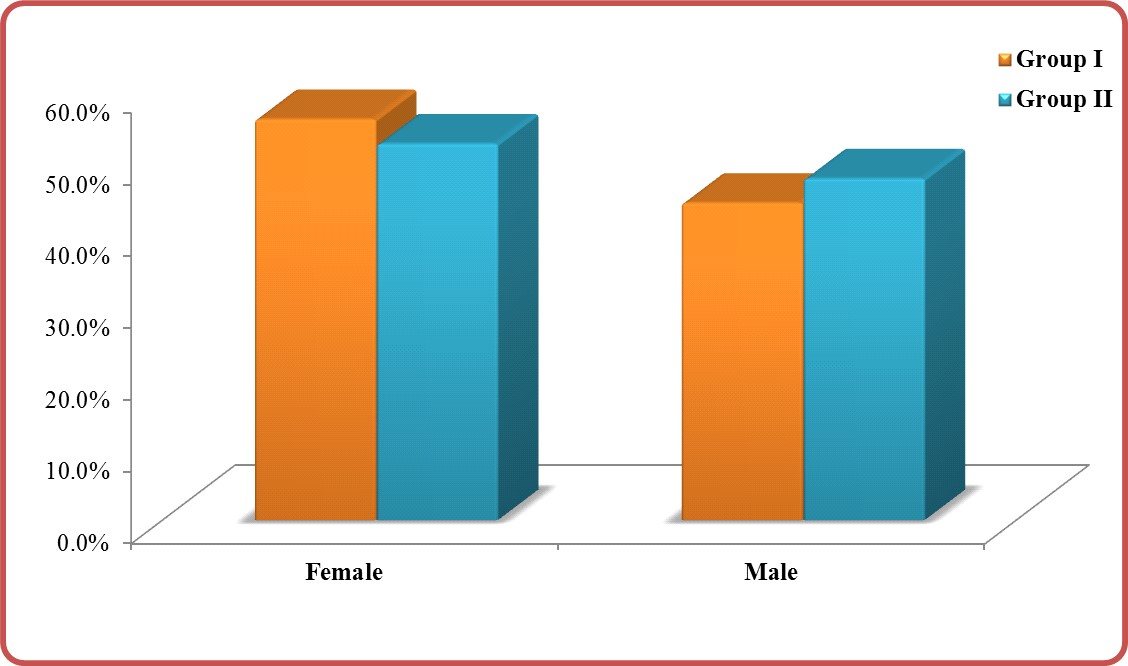
Figure 2: Sex in group I vs. group II.
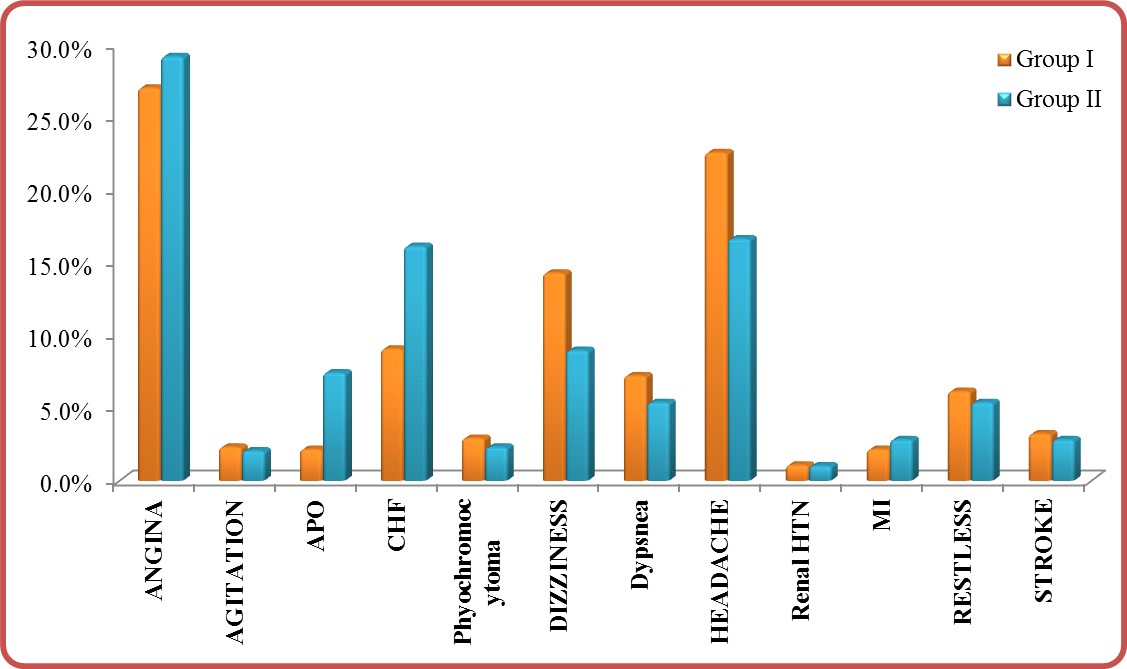
APO: Acute pulmonary edema, CHF: Congestive heart failure, HTN: Hypertension, MI: Myocardial infarction
Figure 3: Hypertension-associated conditions in group I vs. group II.
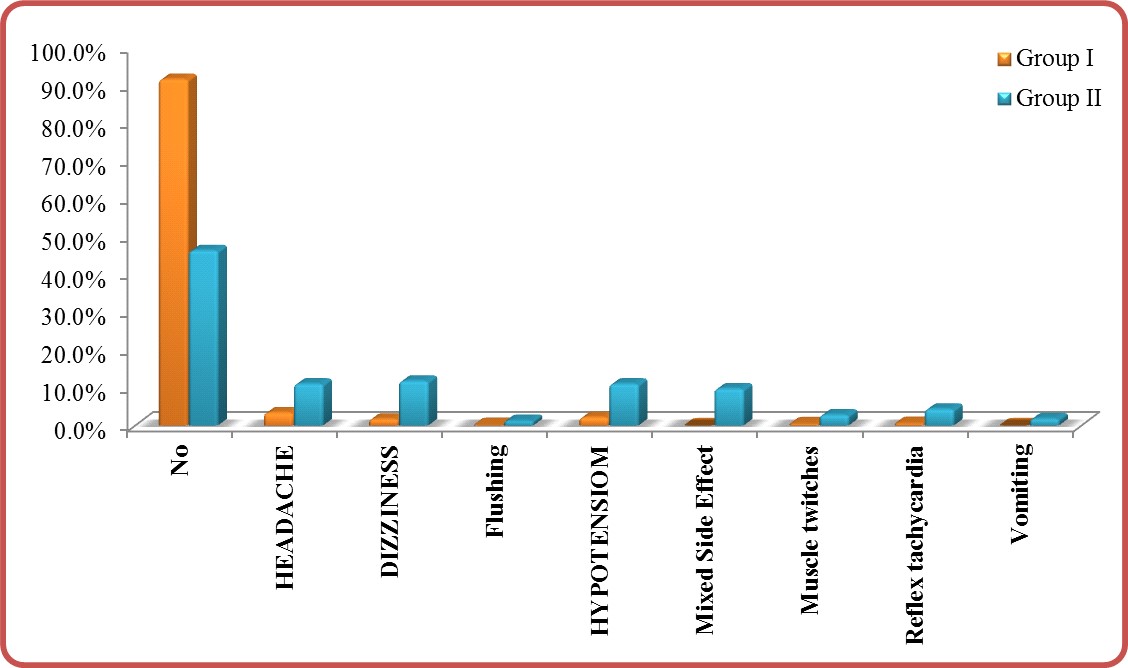
Figure 4: Side effects in group I vs. group II.
BP: Blood pressure
Figure 5: Response in group I vs. group II.
|
Issue |
Definition |
|
Title |
Nitroglycerin in suboptimal versus the remaining doses in hypertensive crises regards propylene glycol; efficacy and safety (Nitroglycerin on trace study) |
|
|
|
|
Estimated Enrollment |
1043 participants |
|
Study Type |
Observational |
|
Observational Model |
Case-only |
|
Time |
Retrospective |
|
Study Start Date |
10-Jul-15 |
|
Estimated Study Completion Date |
10-Jul-19 |
|
Analytic method |
Comparative using both Chi-Squared test and P-value |
Table 1: showing remarks of the study method and data.
|
Group |
Nitroglycerin Concentration: (mg)/Solvent(ml) |
Maintenance dose by µg/min |
Maintenance dose by drops/min |
No. of Patient for each Dose |
Side effects |
|
Group I |
1.25mg/50 ml Solvent |
3.75 |
3 |
68 |
- |
|
2.5mg/50 ml Solvent |
7.5 |
3 |
68 |
- |
|
|
5mg/50 ml Solvent |
15 |
3 |
67 |
Slight headache |
|
|
7.5mg/50 ml Solvent |
22.5 |
3 |
67 |
Muscle twitches |
|
|
10mg/50 ml Solvent |
30 |
3 |
66 |
Headache |
|
|
12.5mg/50 ml Solvent |
37.5 |
3 |
66 |
Dizziness |
|
|
15mg/50 ml Solvent |
45 |
3 |
64 |
Tachycardia |
|
|
17.5mg/50 ml Solvent |
52.2 |
3 |
64 |
Flushing |
|
|
20mg/50 ml Solvent |
60 |
3 |
62 |
Vomiting |
|
|
22.5mg/50 ml Solvent |
67.5 |
3 |
60 |
Headache- Dizziness |
|
|
Group II |
25mg/50 ml Solvent |
75 |
3 |
31 |
Headache- Dizziness |
|
27.5mg/50 ml Solvent |
82.5 |
3 |
30 |
Headache- Dizziness |
|
|
30mg/50 ml Solvent |
90 |
3 |
29 |
Headache- Dizziness |
|
|
32.5mg/50 ml Solvent |
97.5 |
3 |
28 |
Headache- Dizziness |
|
|
35mg/50 ml Solvent |
105 |
3 |
26 |
Mixed 3 Side Effects |
|
|
37.5mg/50 ml Solvent |
112.5 |
3 |
25 |
Mixed 3 Side Effects |
|
|
40mg/50 ml Solvent |
120 |
3 |
24 |
Mixed 4 Side Effects |
|
|
42.5mg/50 ml Solvent |
127.5 |
3 |
23 |
Mixed 4 Side Effects |
|
|
45mg/50 ml Solvent |
135 |
3 |
22 |
Mixed 4 Side Effects |
|
|
47.5mg/50 ml Solvent |
142.5 |
3 |
21 |
Mixed 5 Side Effects |
|
|
50mg/50 ml Solvent |
150 |
3 |
20 |
Mixed 5 Side Effects |
|
|
52.5mg/50 ml Solvent |
157.5 |
3 |
19 |
Mixed ≥ 5 Side Effects |
|
|
55mg/50 ml Solvent |
165 |
3 |
18 |
Mixed ≥ 5 Side Effects |
|
|
57.5mg/50 ml Solvent |
172.5 |
3 |
17 |
Mixed ≥ 5 Side Effects |
|
|
60mg/50 ml Solvent |
180 |
3 |
16 |
Mixed ≥ 5 Side Effects |
|
|
62.5mg/50 ml Solvent |
187.5 |
3 |
15 |
Mixed ≥ 5 Side Effects |
|
|
|
65mg/50 ml Solvent |
195 |
3 |
14 |
Mixed ≥ 5 Side Effects |
|
67.5mg/50 ml Solvent |
202.5 |
3 |
13 |
Mixed ≥ 5 Side Effects |
Table 2: Showing the maintenance doses for nitroglycerine by ug/min in both groups.
|
Variable |
Group I |
Group II |
|
No. |
No: 652 Percent (62.51%) |
No: 391 Percent (37.49%) |
|
Age |
Range: 24-90 median: 66 mean:63.83 |
Range: 24-88 median: 65 mean:63.23 |
|
Sex |
||
|
M |
287 (44 %) |
187 (48 %) |
|
F |
365 (56 %) |
204 (52 %) |
|
1st Hour BP Reduction No. |
576 (88.34%) |
349 (89.25%) |
|
2nd Hour BP Reduction No. |
65 (9.969%) |
34 (8.69%) |
|
3rd Hour BP Reduction No. |
11 (1.68%) |
8 (2.04%) |
|
Side Effects: |
||
|
Headache |
22 (3.37%) |
34 (10.99%) |
|
Dizziness |
11 (1.68%) |
46 (11.76%) |
|
Hypotension |
14 (2.14%) |
43 (10.99%) |
|
Flushing |
1 (0.15%) |
5 (1.27%) |
|
Vomiting |
- (0 %) |
7 (1.79%) |
|
Rebound |
23 (3.5 %) |
15 (3.8 %) |
|
Muscle twitches |
3 (0.46%) |
11 (2.81 %) |
|
Reflex tachycardia |
4 (0.61%) |
17 (4.3 %) |
|
Mixed Side Effects |
8 (1.2%) |
38 (9.7 %) |
|
Hypertension Associate |
||
|
Angina |
176 (26.9%) |
114 (29.15%) |
|
MI |
14 (2.1%) |
11 (2.8%) |
|
CHF |
59 (9.04%) |
63 (16.1%) |
|
APO |
14 (2.1%) |
29 (7.4%) |
|
Stroke |
21 (3.2%) |
11 (2.8%) |
|
Dizziness |
93 (14.26%) |
35 (8.95%) |
|
Headache |
147 (22.5%) |
65 (16.6%) |
|
Phyochromocytoma |
19 (2.9%) |
9 (2.3%) |
|
Renal Hypertension |
7 (1.07%) |
4 (1.02%) |
|
Dypsnea |
47 (7.2 %) |
21 (5.37%) |
|
Agitation |
15 (2.3%) |
8 (2.046%) |
|
Restlessness |
40 (6.1%) |
21 (5.37%) |
|
BP after infusion : 90/70 |
14 (2.14%) |
43 (10.9%) |
|
100/70 |
4 (0.6%) |
9 (0.02%) |
|
110/70 |
155 (23.77%) |
79 (20.2%) |
|
120/80 |
100 (15.33%) |
20 (5.1%) |
|
120/70 |
111 (17.02%) |
62 (15.8%) |
|
130/70 |
71 (10.88%) |
34 (8.6%) |
|
130/80 |
61 (9.35%) |
47 (12.02%) |
|
140/60 |
1 (0.15 %) |
12 (3.06%) |
|
140/70 |
9 (1.38%) |
22 (5.6%) |
|
140/80 |
126 (19.3%) |
63 (16.1%) |
|
SBP |
Range: 180-270 median: 210 mean: 215.67 SD19.9 |
Range: 190-270 median: 210 mean: 212.48 SD 20.8 |
|
DBP |
Range: 120-160 median:140 mean: 139.6 SD 9.766 |
Range: 130-170 median:140 mean: 143.17 SD 12.36 |
|
PP |
Range: 130-150 median:70 mean: 76.25 SD 20.75 |
Range: 20 -140 median:70 mean: 69.41 SD 23.03 |
|
MBP |
Range: 140-190 median: 163.3 mean: 164.99 SD 10.06 |
Range: 150-203 median: 163 mean: 166.21 SD 11.36 |
|
Pulse |
Range: 45-150 median:76 mean: 79.36 SD 15.41 |
Range: 40-145 median:86 mean: 81.42 SD 16.51 |
|
Note: APO: Acute pulmonary edema, BP: blood pressure, CHF: Congestive heart failure, DBP: Diastolic blood pressure, HTN: Hypertension, MBP: Mean blood pressure, MI: Myocardial infarction, PP: Pulse pressure, SBP: Systolic blood pressure. |
||
Table 3: Showing the percent’s and numbers general collective data in both groups of the study.
|
Group |
Age |
T-test |
|
|||
|
Range |
Mean |
± |
SD |
t |
P-value |
|
|
Group I |
24 -90 |
63.833 |
± |
13.074 |
0.719 |
0.472 |
|
Group II |
24 -88 |
63.230 |
± |
13.136 |
|
|
Table 1: Age in group I vs. group II.
|
SEX |
Groups |
||||||
|
Group I |
Group II |
Total |
|||||
|
N |
% |
N |
% |
N |
% |
||
|
Female |
364 |
55.8% |
205 |
52.4% |
569 |
54.6% |
|
|
Male |
288 |
44.2% |
186 |
47.6% |
474 |
45.4% |
|
|
Total |
652 |
100.0% |
391 |
100.0% |
1043 |
100.0% |
|
|
Chi-square |
X2 |
1.138 |
|||||
|
P-value |
0.286 |
||||||
Table 2: Sex in group I vs. group II.
|
Hypertension-associated conditions |
|
Groups |
|||||
|
|
|
Group I |
|
Group II |
|
Total |
|
|
|
|
N |
% |
N |
% |
N |
% |
|
Angina |
|
176 |
27.00% |
114 |
29.20% |
290 |
27.80% |
|
Agitation |
|
15 |
2.30% |
8 |
2.00% |
23 |
2.20% |
|
APO |
|
14 |
2.10% |
29 |
7.40% |
43 |
4.10% |
|
CHF |
|
59 |
9.00% |
63 |
16.10% |
122 |
11.70% |
|
Phyochromocytoma |
|
19 |
2.90% |
9 |
2.30% |
28 |
2.70% |
|
Dizziness |
|
93 |
14.30% |
35 |
9.00% |
128 |
12.30% |
|
Dypsnea |
|
47 |
7.20% |
21 |
5.40% |
68 |
6.50% |
|
Headeache |
|
147 |
22.50% |
65 |
16.60% |
212 |
20.30% |
|
Renal HTN |
|
7 |
1.10% |
4 |
1.00% |
11 |
1.10% |
|
MI |
|
14 |
2.10% |
11 |
2.80% |
25 |
2.40% |
|
Restlessness |
|
40 |
6.10% |
21 |
5.40% |
61 |
5.80% |
|
Stroke |
|
21 |
3.20% |
11 |
2.80% |
32 |
3.10% |
|
Total |
|
652 |
100.00% |
391 |
100.00% |
1043 |
100.00% |
|
Chi-square |
X2 |
39.045 |
|
|
|
|
|
|
|
P-value |
<0.001*
|
|
|
|
|
|
|
Note: APO: Acute pulmonary edema, CHF: Congestive heart failure, HTN: Hypertension, MI: Myocardial infarctio |
|||||||
Table 3: Hypertension-associated conditions in group I vs. group II.
|
Side Effects |
Groups |
Total |
||||
|
|
Group I |
Group II |
||||
|
|
N |
% |
N |
% |
N |
% |
|
No |
597 |
91.6% |
181 |
46.3% |
778 |
74.6% |
|
Headache |
22 |
3.4% |
43 |
11.0% |
65 |
6.2% |
|
Dizziness |
11 |
1.7% |
46 |
11.8% |
57 |
5.5% |
|
Flushing |
1 |
.2% |
5 |
1.3% |
6 |
.6% |
|
Hypotension |
14 |
2.1% |
43 |
11.0% |
57 |
5.5% |
|
Mixed side effect |
0 |
0.0% |
38 |
9.7% |
38 |
3.6% |
|
Muscle twitches |
3 |
0.5% |
11 |
2.8% |
14 |
1.3% |
|
Reflex tachycardia |
4 |
0.6% |
17 |
4.3% |
21 |
2.0% |
|
Vomiting |
0 |
0.0% |
7 |
1.8% |
7 |
0.7% |
|
Total |
652 |
100.0% |
391 |
100.0% |
1043 |
100.0% |
|
Chi-square |
X2 |
292.755 |
|
|
|
|
|
P-value |
<0.001* |
|
|
|
|
|
Table 4: Side effects in group I vs. group II.
|
Response |
Groups |
||||||
|
Group I |
Group II |
Total |
|||||
|
N |
% |
N |
% |
N |
% |
||
|
1st H BP REDUCTION |
576 |
88.30% |
349 |
89.30% |
925 |
88.70% |
|
|
2nd H BP REDUCTION |
65 |
10.00% |
34 |
8.70% |
99 |
9.50% |
|
|
3rd H BP REDUCTION |
11 |
1.70% |
8 |
2.00% |
19 |
1.80% |
|
|
Total |
652 |
100.00% |
391 |
100.00% |
1043 |
100.00% |
|
|
Chi-square |
X2 |
0.616 |
|
|
|
|
|
|
P-value |
0.735 |
|
|
|
|
|
|
|
Note: BP: Blood pressur |
|||||||
Table 5: Side effects in group I vs. group II.
IPCS (2009) Poisons Information Monograph 443. Propylene glycol. Citation: Elsayed YMH (2019) Nitroglycerin in Suboptimal Versus the Remaining Doses in Hypertensive Crises Regards Propylene Glycol; Efficacy and Safety (Nitroglycerin On Trace Study); Retrospective Observational Study. Ann Med & Surg Case Rep: AMSCR-1000021