Journal of Diabetes Management and Metabolism
The Relation of Glycemic Control and Subclinical Hypothyroidism in Saudi Patients with Type 2 Diabetes Mellitus
Khalid S Aljabri1*, Ibrahim M Alnasser Facharatz2, Samia A Bokhari1, Muneera A Alshareef1, Patan M Khan1, Abdulla M Mallisho1, Hesham M AbuElsaoud1, Mohammad M Jalal1, Rania F Safwat1, Rehab El Boraie1, Abdullah A Alamri1, Bushra A Baeshen1, Waleed O Bawzeer1, Mohammad A Melibari1, Emad I Alghannami1, Nawaf K Aljabri3, Bandari K Aljabri4, Turky A Alharthy5
1Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia
2Department of Radiology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia
3Department of Laboratory, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia
4Faculty of medicine, Um Al QuraUniversity, Makkah, Kingdom of Saudi Arabia
5College of medicine, Jeddah, Kingdom of Saudi Arabia
*Corresponding author: Khalid S Aljabri, Department of Endocrinology, King Fahad Armed Forces Hospital, PO Box 9862, Jeddah 21159, Kingdom of Saudi Arabia.
Citation: Aljabri KS, Facharatz IMA, Bokhari SA, Alshareef MA, Khan PM, et al. (2019) The Relation of Glycemic Control and Subclinical Hypothyroidism in Saudi Patients with Type 2 Diabetes Mellitus. J Diabetes Metab Manag: JDMM-100003.
Received Date: 23 April, 2019; Accepted Date: 03 May, 2019; Published Date: 10 May, 2019
Abstract
Background and objective: The association between diabetes and subclinical hypothyroidism (SCH) were reported. Thus, the present study was conducted to find out the relationship between type 2 diabetes mellitus (T2DM) and SCH in Saudi patients with T2DM.
Design: A cross-sectional study was conducted in the Diabetes centre at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia from January 2018 to December 2018. Thyroid stimulating hormone (TSH), free thyroxin (FT4) and HbA1c were measured.
Results: A total of 1156 subjects with T2DM were included in this study. Average age of the study population was 55.1 ± 16.2 years. SCH was present in 121 (10.5%) where females were statistically significant more prevalent than males in patients with HbA1c 7-9 % (p=0.006). There was a trend up as age advanced where females were statistically significant more prevalent than males in the forth decade (p=0.02). SCH was statistically significant present in 8.7, 10.6 and 14.9% in patients with HbA1c <7, 7-9 and >9% respectively (p=0.03). Patients with HbA1c 7-9 and >9% were statistically significant older compared to patients with HbA1c<7 (p=0.02) with females were statistically significant more prevalent in patients between HbA1c <7, 7-9 and >9, (75.0, 60.6% and 62.3%) respectively, p= <0.0001. Only HbA1cwas found to be an independent predictor of SCH (P=0.03). A statistically significant negative correlation was observed between TSH and HbA1c(r= -0.007, P= 0.8). Also, a significant negative correlation was observed between FT4 and HbA1c(r= −0.053, P= 0.1).
Conclusion: SCH is highly prevalent in cohort of Saudis with poorly controlled patients with T2DM.
Keywords: Glycemic control and Type 2 diabetes; Subclinical hypothyroidism
Introduction
Thyroid diseases and diabetes mellitus are the two most common endocrine disorders encountered in clinical practice. Diabetes and thyroid disorders have been shown to mutually influence each other [1,2]. The prevalence of thyroid dysfunction is higher in diabetics than in controls. This has been estimated to be between 10 to 15% in diabetes compared to 6% in the non-diabetic population [3-5].
Because advanced assays enable to measure thyroid hormones more accurately, the incidence of Subclinical hypothyroidism (SCH)is tend to increase in the last few decades [6]. The overall prevalence of SCH is reported to range from 4% to 10% in large general population screening surveys, although it varies with age, sex, and race [7-10]. In type 2 diabetes mellitus (T2DM), prevalence of thyroid disease has been found to be as high as 31%, the most common disorder being subclinical hypothyroidism, followed by subclinical hyperthyroidism, overt hypothyroidism and overt hyperthyroidism [11-13]. SCH is defined as an elevated level of serum thyroid stimulating hormone (TSH) with a normal level of serum free thyroxine (FT4) [14].
It is assumed that T2DM is associated with SCH [1,2]. Previous studies suggested SCH was much more likely in patients with T2DM than general population, and the prevalence was reported to be 2.2% to 31% [11-13,15-19]. However, some investigators reported no differences between groups and more studies are needed to clarify the relationship between T2DM and SCH [17]. With regard to controversy, few studies compared the SCH of patients with T2DM to that of general population in Saudi Arabia. Moreover, little is known about the prevalence of SCH according to glycemic control status in diabetic patients.
The present study is carried out to find out the inter relation between SCH and glycemic status in patients with T2DM in a cohort of Saudi population.
Methods
A cross-sectional study was conducted in the Diabetes centre at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia from January 2018 to December 2018 for a period of 12 months which included 1156 patients who were diagnosed as T2DM on the basis of American Diabetes Association criteria and older or equal to 20 years old [20]. Patients who are pregnant were excluded. TSH was measured with a chemiluminescent immunoassay method (CMIA) (Architect i2000 system, Abbott, USA). Serum FT4 was estimated by radioimmunoassay. The assays have intra- assay precision of 4.3%. TSH levels between 0.22-4.2 mIU/L and Free T4 12.0-22.0 pmol/L were regarded normal [21]. High performance liquid chromatography was used. HbA1c was expressed as percentage. SCH was defined as an elevated TSH >4.2 mIU/l with a normal level of serum FT4 [22]. Subjects with T2DM were divided into three groups by glycemic control: HbA1c < 7%; HbA1c ≥ 7- 9%; and HbA1c > 9%. The total number of cohort were separated on basis of age values into six groups: 20-29 years, 30-39, years 40-49, years, 50-59 years, 60-69 years and ≥ 70 years. The study was approved by the ethical board of King Fahad Armed Forces Hospital.
Statistical Analysis
Data are presented as means ± standard deviation (SD) or numbers (%). Quantitative variables were compared between two groups by using the Student’s test. Differences in categorical variables were analyzed using the chi-square test. Differences between three groups were tested with ANOVA. The relationship between continuous variables was assessed using coefficients of correlation. Logistic regression analysis was carried out to identify the independent predictors of SCH considering age, gender and HbA1c as risk factors and to estimate odds ratio (OR) and 95% CI. The statistical analysis was conducted with SPSS version 23.0 for Windows.
Results
A total of 1156 subjects with T2DM were included in this study. Average age of the study population was 55.1 ± 16.2 years (Table 1). We found 31.7% were male and 68.3% were female. Mean HbA1c (%), TSH and FT4 were 7.4 ± 2.0, 3.0 ± 3.8 mIU/l and 13.7 ± 3.2 pmol/L respectively. SCH was present in 121 (10.5%) where females were statistically significant more prevalent than males in patients with HbA1c 7-9 % (p=0.006) (Figure 1). There was a trend up as age advanced where females were statistically significant more prevalent than males in the forth decade (p=0.02) (Figure 2).
SCH was statistically significant present in 8.7, 10.6 and 14.9% in patients with HbA1c <7, 7-9 and >9% respectively (p=0.03) (Table 2). Patients with HbA1c 7-9 and >9% were statistically significant older compared to patients with HbA1c<7 (p=0.02) with females were statistically significant more prevalent in patients between HbA1c <7, 7-9 and >9, (75.0, 60.6% and 62.3%) respectively, p= <0.0001. TSH and FT4 levels were non-statistically significant different between HbA1c groups.
In order to identify the independent factors affecting SCH, a multivariate regression model was constructed using SCH as the dependent factor. The constructed model is shown in (Table 3). Age, gender and HbA1c were the independent predictors of SCH. In the constructed model, only HbA1cwas found to be an independent predictor of SCH (P=0.03).
A statistically significant negative correlation was observed between TSH and HbA1c (r= -0.007, P= 0.8). Also, a significant negative correlation was observed between FT4 and HbA1c (r= -0.053, P= 0.1).
Discussion
There is a complex interaction between thyroid dysfunction and diabetes mellitus [1-3,11,16,23-26]. SCH or mild thyroid failure is a common problem, with a prevalence of 3-8% in the population without known thyroid disease [9,27]. In the present study among the 1156 subjects with T2DM, SCH was present in 10.5%. Moreover, poor glycemic control was associated with higher prevalence of SCH especially HbA1c > 9%.The prevalence increases with age and is higher in females. These findings are supported by various studies [28-30]. Celaniet al. [3] found that prevalence of thyroid disease in type 2 diabetics was 31.4%, out of which SCH was most common form (48.3%) [11].
In our study we found that patients with HbA1c ≥7 have higher prevalence of SCH. In addition, HbA1c was independent risk factor associated with developing SCH (OR: 1.1, p=0.03). These findings are supported by various studies who stated that de-arrangement in glycemic control influences the thyroid hormone levels [25,29,30].
Hypothyroidism falsely raises HbA1c due to decreased erythropoiesis. Thyroid hormone replacement is associated with decrease in HbA1c level, which is influenced by increased erythropoiesis rather than by changes in glucose level [25]. The presence of both raised and low levels of thyroid hormones in diabetic may be due to modified thyrotropin releasing hormone (TRH) synthesis and release [25]. The hyperglycaemia seen in type- 2 diabetics is known to have negative effect on thyroid function precisely blunting the pituitary TSH response to stimulation by hypothalamic TRH. This may be due to possible alteration of post translational glycosylation of TRH hence affecting its biological activity [31]. T2DM is associated with increased insulin level and C-peptide level. Insulin is an anabolic hormone known to enhance TSH turnover, which is protein in nature. Recently, C-peptide has been shown to enhance Na+/K+- ATPase activity, an action that may also increase protein synthesis. Such an action would induce increased turnover of TSH, a protein hormone [32,33]. Decreased glucose disposal (as compared with euthyroid subjects) has been proved in hypothyroid patients. Hypothyroidism results in unimpaired or decreased liver glucose output thereby compensating for insulin resistance present in peripheral tissues and accounting for the diminished insulin requirement for glycemic control in hypothyroid diabetic patients. Insulin resistance has been also reported in subclinical hypothyroidism, adding one more possible mechanism to the association of sub-clinical hypothyroidism and cardiovascular risk. Taken together, these findings indicate that an elevated level of serum TSH may be associated with fasting hyperglycemia and insulin resistance in patients with HbA1c levels above 9%. The relationship between SCH and T2DM seems to have a complex interdependent interaction, and more studies clarifying the exact mechanisms are still needed.
Our results showed a non-statistically significant different in both males and females in patients with T2DM with SCH between HbA1c groups. Still, detailed molecular mechanisms remain unclear, because sex hormones (such as estrogen, and testosterone) can regulate the thyroid function [34]. The difference in sex hormones may partly explain the sex-difference in the relationship between thyroid hormone levels. However, because levels of sex hormones such as testosterone and estrogen were not measured in this study, further research is needed to explore this issue. In addition, because the sample size was smaller for males (31.7%) than in females (68.3%), the precision and statistical power of the analysis may be lower for males. Further large-scale population studies are required to confirm the above findings.
We aimed to identify the relation of glycemic control and SCH in Saudi patients in hospital-based health care setting. Furthermore, due to the cross sectional nature of this study, the observed population reflects a selected yet comprehensive group of patients rather than the general population. In addition, the current study population may appear limited in size and therefore may underestimate the true relation of glycemic control and SCH in patients with T2DM.
We conclude that despite the limitations of this hospital-based retrospective study, SCH is highly prevalent in cohort of Saudis with poorly controlled patients with T2DM. In the absence of registry data, larger cooperative studies involving diverse population samples from multiple centers could help to provide further information on the true relation nationally.
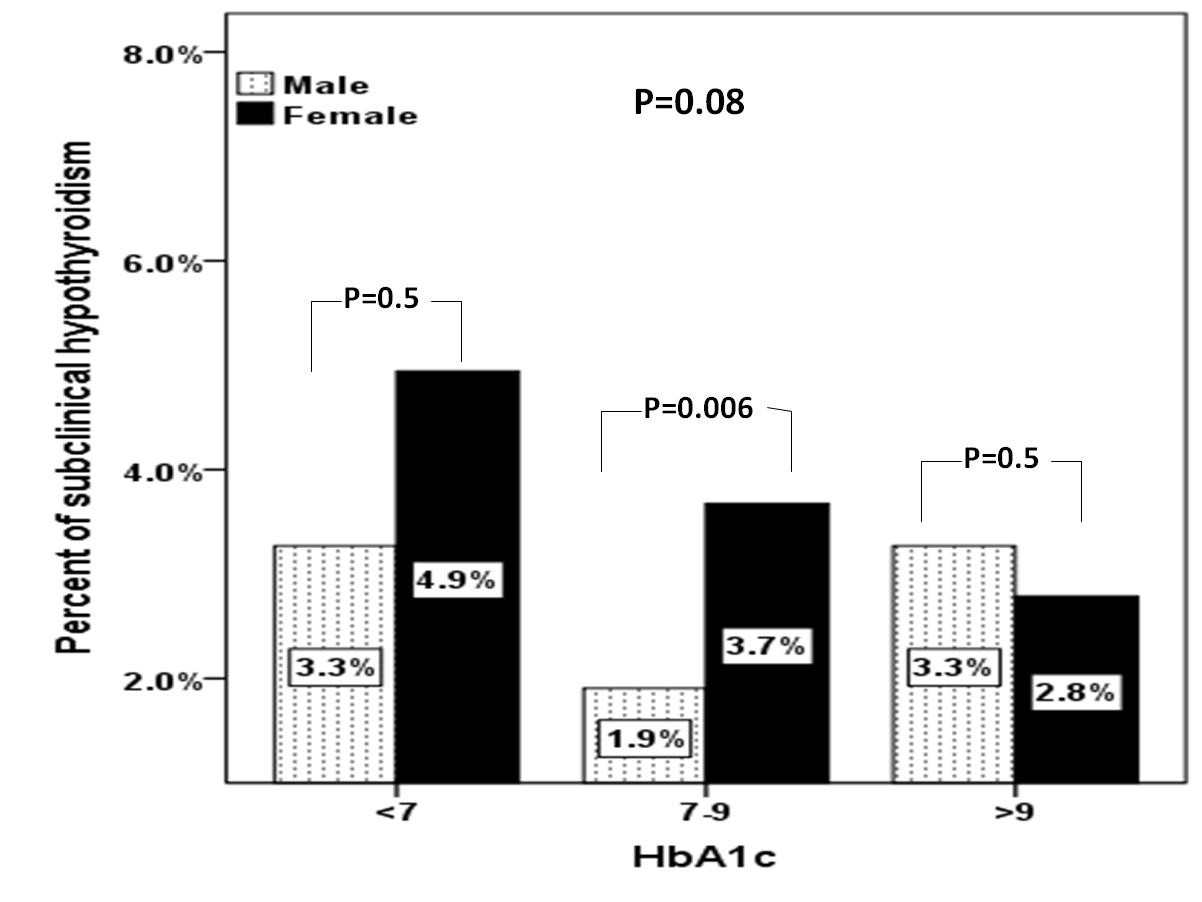
Figure 1: Prevalence of subclinical hypothyroidism between gender in relation to HbA1c categories
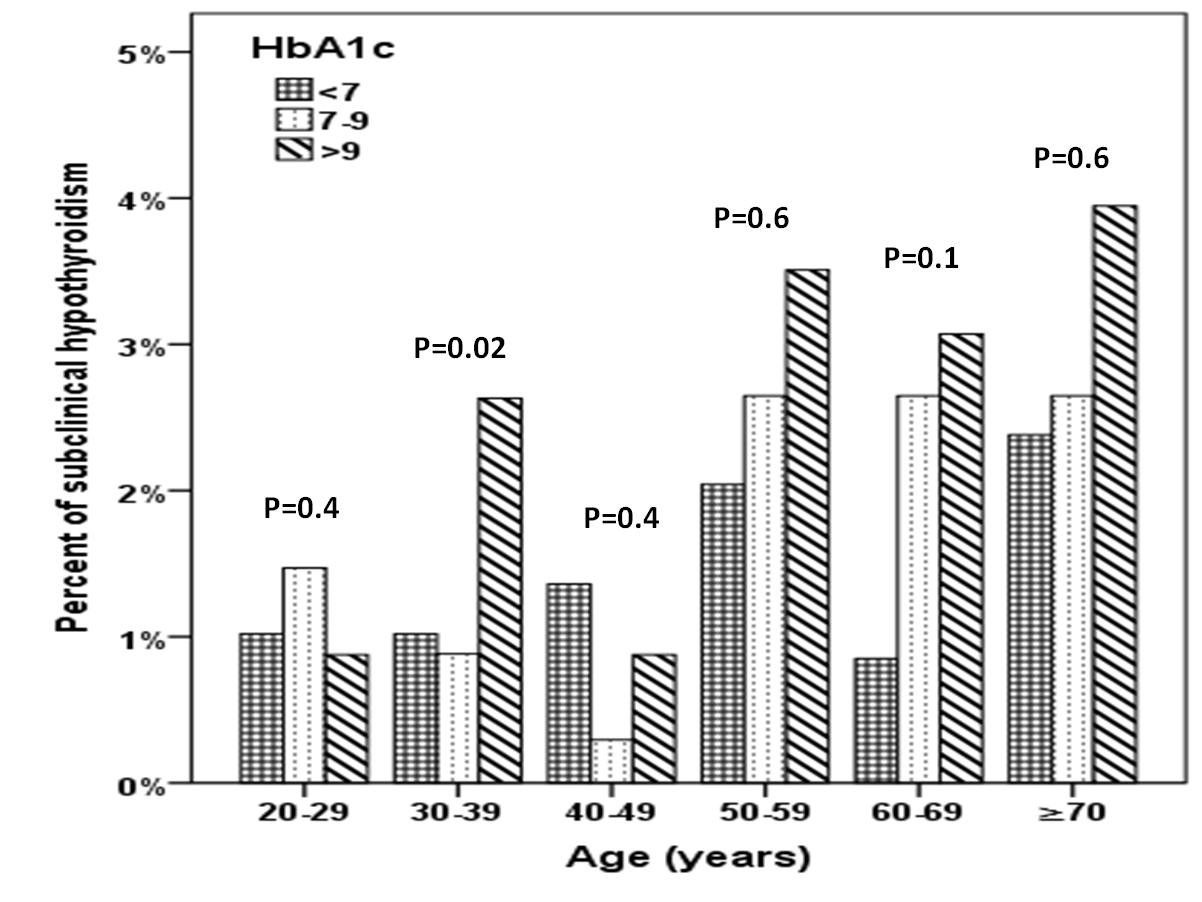
Figure 2: Prevalence of subclinical hypothyroidism between HbA1c categories in relation to age distribution
|
Parameters |
Total ( 1168 ) |
||
|
Age (years) |
55.1 ±16.2 |
||
|
Gender |
Male |
367 ( 31.7 ) |
|
|
Female |
789 ( 68.3 ) |
||
|
HbA1c (%) |
7.4 ±2.0 |
||
|
TSH ( mIU/l) |
3.0 ±3.8 |
||
|
FT4 ( pmol/l) |
13.7 ±3.2 |
||
|
Subclinical hypothyroidism |
121 ( 10.5 ) |
||
Table 1: Base line characteristic of total population
|
Variable |
HbA1c (%) |
P values |
|||
|
<7 |
9-Jul |
>9 |
|||
|
Numbers |
588 (50.9) |
340 (29.4) |
228 (19.7) |
||
|
Age (years) |
53.8 ± 16.0 |
56.9 ± 15.7 |
55.6 ± 17.1 |
0.01 |
|
|
Gender |
Male |
147 (25.0) |
134 (39.4) |
86 (37.7) |
<0.0001 |
|
Female |
441 (75.0) |
206 (60.6) |
142 (62.3) |
||
|
HbA1c (%) |
5.8 ± 0.6 |
7.9 ± 0.6 |
10.7 ± 1.3 |
<0.0001 |
|
|
TSH (mIU/l) |
2.9 ± 4.8 |
3.3 ± 2.9 |
2.8 ± 1.8 |
0.2 |
|
|
FT4 (pmol/l) |
13.9 ± 3.8 |
13.4 ± 2.6 |
13.7 ± 2.4 |
0.05 |
|
|
Subclinical hypothyroidism |
51 (8.7) |
36 (10.6) |
34 (14.9) |
0.03 |
|
Table 2: Characteristic of patients according to HbA1c (%)
|
Parameters |
Coefficients |
95% Confidence interval |
P value |
|
Age (years) |
1.001 |
0.989-1.013 |
0.8 |
|
Gender |
0.678 |
0.439-1.045 |
0.08 |
|
HbA1c ≥ 7 (%) |
1.102 |
1.009-1.203 |
0.03 |
Table 3: Regression analysis using subclinical hypothyroidism as the dependent variable
Citation: Aljabri KS, Facharatz IMA, Bokhari SA, Alshareef MA, Khan PM, et al. (2019) The Relation of Glycemic Control and Subclinical Hypothyroidism in Saudi Patients with Type 2 Diabetes Mellitus. J Diabetes Metab Manag: JDMM-100003.