Insights of Cardiology Open Access
Cardiogenic Shock Management Based by Minimally Invasive Monitoring
Alejandro Castaneda1, Arturo Camarillo1, Joseph Varon2 and Santiago Herrero3*
1Research Assistant, Dorrington Medical Associates, Houston, USA
2Clinical Professor of Medicine, University of Texas in Galveston, Chief Critical Care Services, United Memorial Medical Center, United General Hospital, Houston, USA
3Director of Cardiac Critical Care Department, Adults and Pediatric Post op Cardiac Surgery Unit, Coronary Care Unit, Respiratory Care Unit, Cardiac minimally invasive procedures, The Jilin Heart Hospital, Changchun, Jilin, China
*Corresponding author: Santiago Herrero, Director of Cardiac Critical Care Department, Adults and Pediatric Post op Cardiac Surgery Unit, Coronary Care Unit, Respiratory Care Unit, Cardiac minimally invasive procedures, The Jilin Heart Hospital, 5558 Jingyue St, Changchun, Jilin, China, Tel: +864000120120.
Citation: Castaneda A, Camarillo A, Varon J, Herrero S (2019) Cardiogenic Shock Management Based by Minimally Invasive Monitoring. Insights Cardiol Open Acc: ICOA-100002.
Received date: 27 March, 2019; Accepted date: 15 April, 2019; Published date: 17 April, 2019
Abstract
A fifty-seven-year-old woman, with a large myometrial tumor who underwent a mechanical mitral valve replacement with a left ventricular ejection fraction (EF) estimated at 63% prior to surgery and post operation ranging at 17%. Following surgery, cardiogenic shock (CS) was diagnosed after an electrocardiogram (ECG) monitor that revealed a narrow paroxysmal supra ventricular tachycardia at 165 bpm, as well as, a reduction in central venous oxygen saturation (ScvO2) from 52% to 41%. A cardiac index (CI) of 1.53 L/min/m2 and stroke volume index (SVI) 19.9 ml/m2 was evaluated by pulse contour analysis of cardiac output (PiCCO) as a minor invasive method.
The management was also guided by the assessment of arterial-venous blood gas variables. Volumetric monitoring using the transpulmonary thermodilution technique is useful for assessing preload, particularly the measurement of global end-diastolic volume index (GEDVI) reflecting central blood volume which is important in critically ill patients in shock. The administration of vasoactive drugs and fluid was adjusted to the hemodynamic state of the patient, as soon as new PiCCO information and gas variables were analyzed, helping improve care and enhance a better outcome.
Keywords: Arterial and venous blood gases; cardiac minimally invasive monitoring; cardiogenic shock; pulse contour cardiac output
Introduction
Cardiogenic shock (CS) is commonly known as end organ hypoperfusion due to circulatory failure related to an inadequate supply of oxygenated blood to the tissues [1]. It belongs to the leading causes of morbidity and mortality in patients with acute cardiac conditions [2]. Primarily triggered by ischemic etiologies such as ST-segment elevation myocardial infarction (STEMI) or non-ST-segment elevation myocardial infarction (NSTEMI). Other causes can include pharmacological, ventricular dysfunction, outflow obstruction, acute valvular regurgitation, endocrine, pericardial disease, tachyarrhythmias and bradyarrhythmia [3].
The European Society of Cardiology (ESC) guidelines were the first to classify patients with acute heart failure syndromes (AHFS) into distinct clinical conditions including the cardiogenic shock. However, the ESC classification is complex and is based on pathophysiology, clinical phenotype and disease severity on presentation. This clinical conditions can be differentiated that permit an ideal medical therapy adjusted to each individual (Figure 1) [4].
Clinically this patients present signs such as hypotension, tachycardia, oliguria, cool extremities, and altered mental status. Hemodynamic findings are sustained arterial hypotension with systolic blood pressure below 90 mm Hg or mean arterial blood pressure below 70 mmHg for 30 minutes or longer with or without therapy, cardiac index <2.2 L/min/m2, elevated pulmonary artery occlusion pressure (>15 mm Hg) [5].
Aim of the study
The management of cardiogenic shock has been common in the use of invasive monitoring and treatment models, such as the measurement of pulmonary arterial pressure with the Swan-Ganz balloon flotation catheter and the introduction of supports such as intra-aortic balloon counterpulsation (IABP) during or after cardiac surgery or even short-term mechanical circulatory support in patients who remain severely hypoperfused. Some of these methods has been questioned, although it is still common this kind of management in many cardiac centers. There is a current recommendation of class IIIB for the routine use of IABP in cardiogenic shock [6].
In our case, significant hemodynamic changes and hypoperfusion were revealed after the patient had paroxysmal narrow supraventricular tachycardia, a condition after mechanical valve replacement surgery. The diagnosis and management of the state of cardiogenic shock in this case were the management of arterial and venous gases, as well as the introduction of the pulse contour analysis of cardiac output.
An adequate understanding of the pathophysiology of arterial and venous gases when evaluating the different respiratory and parameters of oxygenation can benefit the monitoring and treatment of patients in shock, avoiding the implementation of other more invasive methods.
Methods and Case Presentation
A 57-year-old, Chinese woman (weight: 44 Kg and height: 147 cm), blood type AB positive (+), with a history of alopecia for the past 20 years, arthritis and cardiac valve disease (of rheumatic cause), presented to our hospital with shortness of breath and fatigue due to severe stenosis (1.0square cm open area) and severe regurgitation of the mitral valve. The echocardiography show a left ventricular ejection fraction (LEVF) of 63% prior of surgery. Left atrial 80 mm; Left ventricular size: 65 mm; Septum size: 0.7 cm and ascending aorta: 29 mm. It was also detected a mild regurgitation of the aortic valve. No tricuspid regurgitation. Pulmonary hypertension estimated 24%. There is no endocavitary thrombi Coronary angiography normal. The patient was in atrial fibrillation prior surgery. Normal nutrition state (BMI: 20.36). Lung function with FVC: 1.31 liters; FVC%: 61.1% FEV1%: 44.41%. PaO2 basal: 76 and SatO2: 96%. Renal function with preoperatory creatinine: 0.52 mg/dl. Hemoglobin: 13.40 gr/l. Hcto: 38.8%. Platelets: 153000 mcL.
Surgery was performed the following day. A central venous catheter was placed leaving the tip in superior vein cava. Arterial blood gases were monitored using a radial arterial line. Severe hypotension was observed during induction supported with milrinone (MIL) with a bolus 3.2 mg and 0.3 mcg/kg/min in continuous perfusion, and norepinephrine (NE) 0.184 mcg/kg/min. Cardio pulmonary by-pass (CPB) duration: 123 minutes. Aortic cross-clamp: 79 min. Mechanical (29MJ, Master Series, SJMTM) mitral valve replacement was performed in addition to an atrial appendage procedure. During operation was necessary to transfuse 2 units of blood on by-pass, 5 gr of tranexamic acid, one gram of fibrinogen and 690 ml of frozen fresh plasma because a complex procedure with a moderate per operatory bleeding. No pacing. Sinus rhythm prior admission in the ICU. Cefuroxime was the prophylactic antibiotic given. Blood loss of 460 ml during first 12 hours in the ICU was observed.
On admission to the intensive care unit (ICU) for follow up care, her vitals were stable (Table 1 and 2). In sinus rhythm, Physical examination revealed a large meso/hypogastric mass which was later identified by abdominal echography as a possible myoma, measuring 20 × 10 cm in hypogastrium (no identified before to the surgery).
During the patient's stay in the ICU the following procedures were performed: sedation under propofol (2.5 mg/kg/hour) and morphine (2 mg/hour) in continuous perfusion, vital signs monitoring with a 78 mmHg of mean arterial pressure (MAP), heart rate (HR): 86 bpm. Supported with NE 0.08 mcg/kg/min and MIL 0.4 mcg/kg/min. Extraction of arterial blood gases within admission, at 3 and 6 hours being reported following oxygen parameters: partial arterial oxygen pressure (PaO2); arterial venous oxygen content (Ca-vO2); arterial-venous carbon dioxide delta (a-vCO2); central venous oxygen saturation (ScvO2); respiratory index: (A-aO2/PaO2); PaO2/FiO2 index (PaFi) or alveolar oxygen gradient and O2ER or extraction of oxygen. The Pulse Contour Cardiac Output (PiCCO) technology using a PiCCO catheter (Maquet Holding B.V. & Co. KG, Germany) was placed in the patient for monitoring the cardiac output (CO), cardiac function index (CFI), extravascular lung water index (EVLWI), intrathoracic blood volume index (ITBVI) and global-end-diastolic volume index (GEDVI) when hemodynamic deterioration occurs.
Complete blood count revealed platelets of 77000/mcL, red blood cells 3.430.000/ml. Plasma creatinine was 0.65 mg/dl, and other biochemical variables were unremarkable. Arterial-venous gases were obtained acquiring samples from superior vein cava and arterial line, where values of Ca-vO2: 4.71 mL/100 mL, delta a-vCO2: 8 mmHg, O2ER: 47.19%, RI: 3.96, PaFi: 118 mmHg, SvO2: 46% and lactate 3.7 mmol/L. This data showed the interpretation of a high metabolism state at admission (high level of delta a-vCO2 plus high O2ER and lactate level > 2.0 with normal Ca-vO2 gradient).
Pressure-regulated volume control (PRVC) ventilation was applied at a fraction of inspired oxygen (FiO2) of 50%, and positive end-expiratory pressure (PEEP) of 5 cm H2O. Follow-up chest X-Ray revealed a large cardiomegaly (Figure 2).In the next few hours, ScvO2 increased gradually reaching 52% after two units of packaged red blood cells that were given due to low hemoglobin (Hb) of 8.4 and hematocrit (Hcto) of 27%.
Reaching the 17-hour mark of ICU care, ScvO2 dropped to 41%, creatinine 70.31 mmol/L, B-type natriuretic peptide (BNP) 2.68 pg/mL and had a fluid balance of +2020, weaning her off NE at 0.04 mcg/kg/min. MIL continued being administrated at 0.3 mcg/kg/min. Arterial-venous gases revealed CavO2 of 6.32, a-vCO2 14, RI 1.13, PaFi 250 mmHg, O2ER 57.04 and ScvO2 41%. The interpretation for this oxygen parameters was a severe hypodynamic state (post-operatory low cardiac output syndrome due a high level of delta a-vCO2>5; O2ER > 32%; Ca-vO2 gradient > 5) although lactate determination was quite normal: 2.2 mmol/L and pH: 7.43. Follow-up echo-cardiogram (ECHO) at the 18 -hour mark revealed the existence of a very poor heart function (LVEF: 17%).
Few minutes after ECHO, the ECG monitor revealed a narrow paroxysmal supraventricular tachycardia at a rate of 165 bpm, lowering mean arterial pressure (PAM): 54 mmHg; urine-output (10 ml after first hour of event) and oxygen arterial saturation (SatO2) was 89%. The narrow paroxysmal supraventricular tachycardia appeared up to two times not being sustained for more than 30 seconds, but with severe hemodynamic instability although electrical cardioversion was not taken into consideration as management to prevent further ventricular damage, nor the implantation of an intra-aortic balloon pump to increase BP, due to possible ischemia and other procedure complications. No previous and posterior changes on ST-T on the ECG were observed. Not was given amiodarone because the severe low blood pressure. Placement of a PiCCO monitoring was considered the optimal approach, which showed the following data (Table 1): CI: 1.53 L/min/m2, stroke volume index (SVI): 19.9 ml/m2, systemic vascular resistance index (SVRI): 2,910 dyn/sec/cm-5m2; EVLWI: 33.6 mL/kg, ITBVI: 1039 mL/m2; GEDVI: 832 mL/m2 and CFI: 1.8 1/min. and stroke volume variation (SVV): 14%, being 8-9% the normal range.
Arterial-venous gases after PiCCO data revealed CavO2 of 5.69, a-vCO2 12, RI 2.95, PaFi 150 mmHg, maximal oxygen consumption index (iVO2) 95, inadequate oxygen delivery index (iDO2) 175, O2ER49.68% and ScvO2 47% with lactate levels reaching 5.4 mmol/L and BNP at 6.65 pg./mL presenting metabolic acidosis. The interpretation for this oxygen parameters was a very severe hypodynamic state (post-operatory low cardiac output syndrome, cardiogenic shock) due a high level of delta a-vCO2>5; O2ER > 32%; Ca-vO2 gradient > 5, lactate >2.2 mmol/L and pH: 7.3.
Results and Management
These results declared the patient in a state of cardiogenic shock which was managed conservatively. A very severe post-operatory left ventricular dysfunction (low cardiac output syndrome) linked to the arrhythmia appeared was the triggering cause of cardiogenic shock.
Based fundamentally on results from PiCCO, CI, EVLWI also SVV and the respiratory and oxygen parameters (Ca-vO2; delta avCO2; O2ER; lactate and pH) was necessary to increase the doses of norepinephrine (0.04 to 0.2 mcg/kg/min) to get a MAP of > 65 mmHg. Milrinone (0.5- to 0.7 mcg/kg/min), digoxin (0.5 mg) followed of 0.25 mg/day and levosimendan (0.05-0.1 mcg/kg/min) where given for better contractilityavoiding the administration of dobutamine due to its arrhythmogenic profile in a potential state of relative hypovolemia and its greater oxygen consumption. Afterwards nitroprusside was infused (0.5 to 1.0 mcg/kg/minute at 5-minute intervals) as an arterio-venous dilator to improve afterload. Carvedilol a very low dose (2.5 mg bid) asβ-blocker to decrease oxygen demand from the heart and albumin at 5%(25 gr albumin in 500 ml of saline 0.9%) in continuous infusion every 12 hours to regulate the oncotic pressure. Also furosemide (3.3 mg/hour, without bolus) was given. This management is based on the difference founded between high EVLWI (i.e. pulmonary edema)versus SVV that showed values ??higher than 10% (14%), which means a low preload in patients mechanically ventilated allows the accurate prediction and monitoring the cardiac index in response to volume administration without overloading fluids in a short time.
After the first 4 hours of the episode of shock, the patient show a progressive improvement of the CI: 1.65L/min/m2, decreasing EVLWI: 28.2 mL/kg, CFI: 2.2 l/min, however the SVI does not change. No more episodes of tachycardia.
The following days PiCCO and arterial-venous gases were taken for evaluation of patient’s prognosis (See table 1, 2).No severe multiorgan disfunction was observed except a moderate liver failure due an elevation of liver enzymes and hyperbilirubinemia. Was performed follow up from echocardiographic point of view with determinations of the LVEF between 18-19% at least for 9 days more.
Due to an evident improvement of the respiratory gases the patient was weaned off mechanical ventilation on the initial sixth day of postoperative evolution (RI: 0.49; Qs/Qt 3.49, CavO2: 4.83 and PaO2: 110 with an FiO2: 0.35 (PaO2/FiO2: 314.3). On her 11th day in ICU, the patient was finally discharged to floor care, with last PiCCO and arterial-venous gases showing significant hemodynamic respiratory/oxygen improvement, being last LVEF observed of 35%.
Discussion
Cardiogenic shock (CS) represents a life threatening emergency defined as persistent hypotension and tissue hypoperfusion due to cardiac dysfunction (primarily caused by myocardial damage, cardiac valve dysfunction, or arrhythmia) in the presence of adequate intravascular volume and left ventricular filling pressure, leading to ischemia of several organs in addition to a systemic inflammatory response that increases morbidity and mortality [1-6]. Organ perfusion depends essentially on blood flow and therefore on cardiac function. Cardiac function is related to the three main physiological determinants: preload, contractility, and afterload that determine stroke volume and finally cardiac output [7].
Rapid assessment of the history, physical examination, and chest radiograph is mandatory, recognizing the signs of heart failure, pulmonary edema (sometimes with clear lung fields on examination), and tissue hypoperfusion, manifesting as low blood pressure, rapid heart rate, agitation, confusion, oliguria, cyanosis, and cool and clammy skin. Appreciating the electrocardiographic signs of acute myocardial ischemia, myocardial infarction, new left bundle branch block, and arrhythmias is critical. A rapid echocardiographic assessment is key [3]. The echo-Doppler study will assess regional and global left ventricular function, right ventricular size and function, the presence of mitral regurgitation and other valve abnormalities, pericardial effusion, and possible septal rupture. Differential diagnoses to consider in patients with CS include hemorrhage, sepsis, aortic dissection, and massive pulmonary embolism.
Patients must be assessed regarding the need for sedation, intubation, and mechanical ventilation in order to correct hypoxemia and reduce the work of breathing [8].
In cardiogenic shock, it is really necessary to know if the preload is adequate, since even regardless of the type of shock it is considered that there is initially a relative hypovolemia [9]. The initial medical therapy includes intravenous fluid challenge for patients with significant hypotension, if there is no evidence for pulmonary edema or significant elevation of jugular venous pressure. However the monitoring of cardiac filling pressures, such as central venous pressure (CVP) and pulmonary artery occlusion pressure (PAOP) is unreliable for assessing cardiac preload in patients mechanically ventilated [10]. In contrast, volumetric monitoring using a transpulmonary thermodilution technique has been shown to be useful for reliably assessing preload, particularly the measurement of global end-diastolic volume index (GEDVI) reflecting central blood volume, although these parameters do not allow the additional prediction of fluid responsiveness [7-11].
PiCCO-Technology is based on transpulmonary thermodilution (TPTD) and continuous pulse contour analysis approaches. PiCCO is a minimally invasive technique and allows the monitoring of beat-by-beat cardiac output. In addition, volume status and pulmonary edema can be monitored, as well as the hemodynamic status. Hemodynamic management and appropriate fluid therapy remain a challenge in critically ill patients. The PiCCO system allows a further measuring extravascular lung water index (EVLWI) monitoring seems to offer a reliable tool for assessing potential pulmonary edema [12], although we should consider that the elevation of the EVLWI does not mean on fact hypervolemia. A hypovolemic state would be recognized by evaluating the SVV when value it is greater than 10%.SVV represents the variation (as a percentage) of SV during the ventilation cycle and is assessed with following equation: SVV (%) = (maximum SV-minimum SV) / mean SV, where the maximum and minimum SV are mean values of the four extreme values of SV during a period of 30 s, and the mean SV is the average value for this time period [13]. Fully sedated patients with mechanical ventilation, sinus rhythm, or pacing in a fixed mode and unchanged catecholamine management are prerequisites for proper use of this hemodynamic monitoring tool [13].One study compared the effect of treatment on cardiac surgery patients, based on PiCCO monitoring to the historical control and found that PiCCO-based fluid management was able to reduce the number of days on vasopressors and shorten the length of stay in ICU [14].
Current literature suggests that indications for ABG analysis should be based on the clinical assessment of the patient. Venous and arterial puncture for venous and arterial blood gases analysis is a minimal and basic invasive procedure also for introducing of the central venous catheter (CVC) placed in superior vein cava and PiCCO’s catheter placed in the common points of arterial cannulation. Central venous O2 saturation (superior vena cava, ScvO2) can be monitored with less patient risk than mixed venous O2 saturation (pulmonary artery, SvO2). SvO2 and ScvO2 over a broad range of cardiorespiratory conditions, including hypoxia, hemorrhage, and resuscitation in anesthetized dogs were well correlated [15].
Arterial blood gas samples are frequently obtained for reasons such as change in ventilator settings, a respiratory or cardiac event, and as routine testing. The use of arterial and venous gases can identify up five respiratory parameters (Ca-vO2; delta a-vCO2 and O2ER; pH and lactate) of which the first three can help assess the hemodynamic state [16]. The assess of Ca-vO2 (CaO2-CvO2)> 7% (normal value 3.6-5.1%); delta a-vCO2 (PaCO2 -PvCO2) >13% (normal value 5%) and O2RE > 37% (normal range 22-32%) can determinate a hypodynamic state by low cardiac output (CO). We can also analyze different metabolic states according to the results, as well as the time when these parameters are out of range and can worsen the hemodynamic process with the elevation of lactate levels and metabolic acidosis. The prompt determination along with the echocardiographic and PiCCO monitoring can establish a optimal and tailoring treatment and improve the prognosis of the CS [16].
About pharmacological therapies with the use of inotropic drugs, digoxin has been shown to help in weaning off the mechanical circulatory support and inotropic agents in patients with left ventricular dysfunction [17].
Dobutamine is an inotrope with arterial dilator properties, can be used in patients with less severe hypotension or can be combined with vasopressors to improve cardiac output [18], when preload is normal.
Levosimendan exhibits calcium-dependent binding to the N-terminal domain of cardiac troponin C (TnC) with a higher affinity at high calcium concentrations and lower affinity at low calcium concentrations [19]. The positive inotropic effect is obtained without increasing intracellular calcium concentration or without a significant increase in myocardial oxygen demand, usually seen with other inotropes [20]. Several clinical observations reveal that levosimendan improve hemodynamics even in patients with cardiogenic shock if it is combined with catecholamines to maintain adequate perfusion pressure [19]. There are not enough studies to indicate the benefit of levosimendan in patients with cardiogenic shock of other etiologies besides acute myocardial infarction, but there is more frequent find several contribution to the clinical development, in a specific indication as the low cardiac output syndrome (LCOS) after extracorporeal circulation during heart surgery [21]. LCOS was defined as the need for inotropic or mechanical circulatory support for >24 hours postoperatively [22].
Intravenous amiodarone can be given for patients with severe arrhythmia if not severe hypotension. Physicians using current formulations of intravenous amiodarone should be aware of it life-threatening acute adverse effects such as severe hypotension secondary to cardiogenic shock [23].
The use of beta blockers and nitrates should be avoided in the acute phase, however if the patient has a low EF with a significant tachycardia, carvedilol combined with levosimendan could be used to help improve the cardiac output even in acute phase [24].
Conclusion
We provide a case in which the medications were adjusted as soon as the values of PiCCO and venous-arterial gases were analyzed after the insult. The use of advanced hemodynamics to evaluate the EVLW and the SVV are linked to the parameters of the respiratory gases (arteriovenous), since the minimally invasive monitoring shows a great importance in the treatment of critical patients with shock, which helps to improve the patient care.
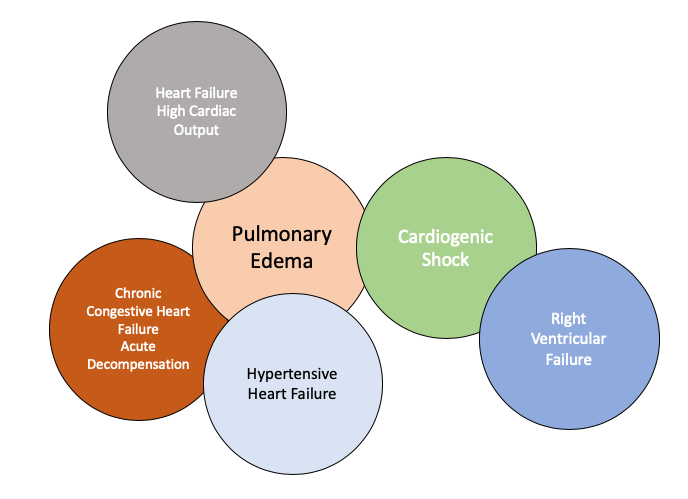
Figure 1: Phenotypes of the heart failure.
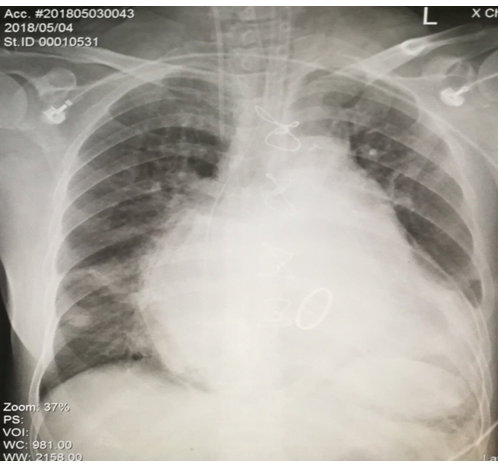
Figure 2: Chest X-Ray (PA) where an enlarged cardiac silhouette is observed.
|
Hemodynamic parameters |
|||||||||||
|
Date |
Day 0 |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
|||||
|
Hour |
12:23 (*) |
16:24 |
20:57 |
7:38 |
18:57 |
6:36 |
7:08 |
14:51 |
8:46 |
7:30 (**) |
|
|
CFI |
1.8 |
2.2 |
2.7 |
2.9 |
2.6 |
2.6 |
3 |
2.6 |
2.4 |
2.7 |
|
|
CI |
1.53 |
1.65 |
2.11 |
2.11 |
2.35 |
2.38 |
2.32 |
2.11 |
1.86 |
2.3 |
|
|
SI |
19.9 |
19.2 |
25.1 |
28.4 |
30.1 |
28 |
30.1 |
24.9 |
23.2 |
22.8 |
|
|
SVRI |
2919 |
2852 |
2127 |
2013 |
1772 |
1946 |
2003 |
2536 |
2325 |
2468 |
|
|
LCWI |
1.5 |
1.7 |
2 |
1.9 |
2.1 |
2.5 |
2.2 |
2.4 |
1.9 |
2.7 |
|
|
LVSWI |
19.8 |
19.6 |
23.5 |
25.5 |
27.4 |
29.4 |
29 |
28.1 |
24.3 |
27 |
|
|
EVLWI |
33.6 |
28.2 |
28.4 |
24.1 |
22 |
19.9 |
25 |
24.4 |
26.7 |
17.2 |
|
|
ITBVI |
1039 |
941 |
963 |
902 |
1089 |
1120 |
966 |
982 |
957 |
1063 |
|
|
GEDVI |
832 |
753 |
771 |
722 |
871 |
896 |
773 |
786 |
766 |
851 |
|
|
PVPI |
5.4 |
5.2 |
4.9 |
4.4 |
3.3 |
2.9 |
4.2 |
4 |
4.6 |
4.2 |
|
|
Milrinone |
0.3 |
0.3 |
0.7 |
0.7 |
0.6 |
0.6 |
0.5 |
0.5 |
0.5 |
0.1 |
|
|
Norepinephrine |
0.04 |
0.2 |
0.2 |
0.2 |
0.3 |
0.27 |
0.12 |
0.12 |
0.12 |
0.02 |
|
|
(*) After Narrow Supraventricular Tachycardia (**) Extubating day |
|||||||||||
Table 1: Hemodynamic changes during the stay in the patient's ICU. Medication rate expressed in mcg/kg/min. Cardiac function index (CFI), cardiac index (CI), stroke index (SI), systemic vascular resistance index (SVRI), left cardiac work index (LCWI), left ventricular cerebrovascular work index (LVSWI), index of pulmonary extravascular water (EVLWI), intrathoracic blood volume index (ITBVI), global end-diastolic volume index (GEDVI), pulmonary vascular permeability index (PVPI).
|
Respiratory and Oxygen Parameters |
||||||||||||
|
Date |
Admission |
Day 0 |
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
|||||
|
Hour |
13:45 |
5:00 |
10:30 (*) |
12:45 |
21:00 |
18:10 |
6:30 |
6:30 |
15:00 |
6:30 |
6:30 (**) |
|
|
PaO2 |
59 |
75 |
75 |
55 |
74 |
84 |
86 |
90 |
119 |
92 |
110 |
|
|
Ca-vO2 |
4.71 |
6.67 |
5.69 |
6.32 |
5.28 |
5.13 |
5.48 |
5.75 |
5.54 |
5.11 |
4.83 |
|
|
delta a-vCO2 |
8 |
14 |
12 |
14 |
6 |
11 |
11 |
9 |
12 |
12 |
8 |
|
|
Qs/Qt (Shunt) |
13.70 |
3.93 |
11.07 |
7.79 |
6.5 |
6.19 |
5.66 |
5.27 |
4.27 |
6.05 |
3.49 |
|
|
PaO2/FiO2 |
118 |
250 |
150 |
137.5 |
211.42 |
240 |
245.71 |
257.14 |
340 |
262.85 |
354.83 |
|
|
RI |
3.96 |
1.13 |
2.95 |
3.04 |
1.6 |
1.26 |
1.19 |
1.11 |
0.64 |
1.12 |
0.49 |
|
|
O2 RE |
47.19 |
57.04 |
49.68 |
55.22 |
44.55 |
45.9 |
48.97 |
46.5 |
46.49 |
45.17 |
39.36 |
|
|
Lactate |
3.7 |
2.2 |
5.4 |
5.7 |
1.5 |
1.3 |
1 |
1 |
1.2 |
0.9 |
1.1 |
|
|
pH |
7.35 |
7.43 |
7.31 |
7.3 |
7.44 |
7.5 |
7.5 |
7.53 |
7.54 |
7.51 |
7.48 |
|
|
Balance (ml) no cumulative |
|
+2020 |
|
|
|
-1670 |
-1238 |
-683.8 |
|
-952.4 |
+7 |
|
|
Urine Output (ml/kg/h) |
|
1.52 |
1.13 |
0.345 |
0.58 |
1.2 |
3.2 |
2.45 |
|
2.14 |
2.03 |
|
|
(*) Initial Narrow Supraventricular Tachycardia (**) Extubating day |
||||||||||||
Table 2: Respiratory and oxygen parameters during the stay in the patient's ICU. Medication rate expressed in mcg/kg/min. Arterial oxygen partial pressure (PaO2), arterial-venous oxygen content difference (Ca-vO2), delta arterial-venous carbon dioxide (a-vCO2), venous shunt nomogram or intrapulmonary shunt (Qs / Qt), oxygen gradient alveolar-arterial (PaO2 / FiO2 or Pa/Fi), respiratory index (RI), Oxygen extraction (O2RE); hydrogen potential (pH).
Citation: Castaneda A, Camarillo A, Varon J, Herrero S (2019) Cardiogenic Shock Management Based by Minimally Invasive Monitoring. Insights Cardiol Open Acc: ICOA-100002.