Journal of Obstetrics and Gynecological Problems
Essure-Attributed Symptoms: Follow-up of a Large Cohort after Device Removal: A Retrospective Nested Case-Control Study
Camil Castelo-Branco1*, Jorge Duro-Gómez2, Marina Romero-Domínguez2, Ana Belén Rodríguez-Marín3, Balbino Povedano-Cañizares2 and Iuliia Naumova1
1Institute Clinic of Gynecology, Obstetrics and Neonatology, Faculty of Medicine-University of Barcelona, Hospital Clinic-Institut d´Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS); Barcelona, Spain
2Department of Gynecology and Obstetrics, Reina Sofía University Hospital, Cordoba, Spain
3Department of Gynecology and Obstetrics, HospitalSan Juan de Dios, Córdoba, Spain
*Corresponding author: Camil Castelo-Branco, Institute Clinic of Gynecology, Obstetrics and Neonatology, Faculty of Medicine-University of Barcelona, Hospital Clinic-Institut d´Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS); Barcelona, Spain. Tel: +34932275436, Fax: +3993227932.
Citation: Castelo-Branco C, Duro-Gomez J, Romero-Dominguez M, Rodriguez-Marin AB, Povedano-Canizares B, et al. (2019) Essure-Attributed Symptoms: Follow-up of a Large Cohort after Device Removal: A Retrospective Nested Case-Control Study. J Obstet Gynecol Probl: JOGP-100002.
Received Date: 07 March, 2019; Accepted Date: 22 March, 2019; Published Date: 01 April, 2019
Abstract
Objective: To analyse the reasons for Essure® removal in a large cohort of patients.
Design: Retrospective 15-year study.
Setting: Office hysteroscopy unit in a teaching hospital.
Methods: From 2003 to 2015, 8024 Essure® device insertions were performed in a single centre in Córdoba (Spain). Of these, 156 patients (1.89%) presented adverse events requiring device withdrawal (SG) A subset of 156 women matched for age, clinical characteristics and time since insertion were used for case-control comparisons (CG). In 47 cases, the device had already been surgically removed. Three months later, patients were questioned regarding symptoms and their general condition.
Main Outcome Measure(s): Pre and post-operative adverse events, early postoperative and late complications (after the initial 3 months) and patients’ satisfaction after procedures.
Results: In the SG, 23.08% (36) of the patients were allergic to nickel compared to only 1.28% (2) in the CG (p<0.01). No differences were found in insertion difficulty or pain or related to the time with the device. Surprisingly, satisfaction was higher at 3 months in the SG compared to the CG (100% vs. 90.90 %, respectively (p=0.01)). Currently, 58.61% (93) of the SG and 1.28 % (2) of the CG report pelvic pain as the most bother some symptom (p<0.01). The SG described abundant menstrual bleeding, allergy, asthenia, polyarthralgia and urinary tract infection. Of the 47 patients who had already undergone surgical device removal, 78.72% (37) reporting feeling well/very well, and 85.10% (40) felt better than before surgery. However, up to 93.67% (43) still had some complaints.
Conclusion: Appropriate patient selection and careful preoperative assessment are required. Women should be informed about the benefits and risks of hysteroscopy and traditional sterilisation.
Keywords: Essure removal; Hysteroscopic sterilisation; Laparoscopic salpingectomy; Pelvic pain; Surgical outcomes; Symptom resolution
Tweetable Abstract
Following Essure® insertion some women present symptoms, including pain, leading to device removal.
Introduction
The Essure® device (Bayer HealthCare Pharmaceuticals, Leverkusen, Germany) was approved by the US Food and Drug Administration (FDA) as a method of female sterilisation in 2002. The device is introduced into the isthmus of the uterine tube by ambulatory hysteroscopy [1]. Imaging tests are usually performed after 12 weeks to assess the location of the device. In general, after insertion, the users are very satisfied with the procedure, being highly effective and with a pregnancy rate of 0.15% [2-4].
Since 2013 some patients have referred different symptoms that appear after the insertion of the device and a small percentage of them subsequently choose to have the Essure® inserts removed due to regret or perceived side effects. Complications and complaints associated with the micro inserts include inadequate placement, unplanned pregnancy, infection, and chronic pelvic pain. However, most of the data is from case-reports or from short series and, in addition, there is scant evidence related to the improvement in the symptoms after surgical removal of the Essure inserts. In 2016, the FDA issued a warning recommending the need for post-marketing studies in which gynaecologists identify and record the adverse events referred by their patients [5]. Following this recommendation, we designed the present study with the aim of analysing the causes leading to surgical removal of the Essure® device in a large cohort of patients.
Methods
Patients: From 2003 to 2015, 8024 Essure® device insertions were carried out in a single centre (Hospital Reina Sofía, Cordoba, Spain). The insertion and follow-up procedures have been described elsewhere [1,2]. Three months after device insertion, a survey was conducted to detect possible adverse events and to know the degree of patient satisfaction with the device [3]. Among this sample, from December 2015 to May 2017, 156 (1.89%) patients presented one or more adverse event (AE) probably related to the Essure® device and requested its removal. In 47 cases the Essure® device had already been removed. Additionally, a subset of 156 women taken from the entire sample and matched for age, time of insertion and clinical characteristics were used for case-control comparisons.
Essure Micro-insert: The Essure micro-insert is a spring-like device that consists of a stainless-steel inner coil, a nickel titanium expanding outer coil, and polyethelene terephthalate (PET) fibers. The PET fibers are wound in and around the inner coil. The micro-insert is 4 cm in length and 0.8 mm in diameter in its wound down configuration. When released from the delivery system, the outer coil expands to 1.5 to 2.0 mm in diameter to anchor the micro-insert in the varied diameters and shapes of the fallopian tube. The spring- like device is intended to provide the necessary anchoring forces during the acute phase of device implantation during which time the PET fibers stimulate tissue in-growth into the coils of the Essure® micro-insert and around the PET fibers.
Protocol: A comprehensive clinical history was obtained including personal background, allergies, symptoms and the reason why they considered the symptoms to be related to the device. Women requesting device removal underwent laparoscopic bilateral salpingectomy. Three months after device removal, a structured interview was conducted with the aim of knowing the symptoms and feelings after device withdrawal.
Adverse Event Monitoring: An adverse event was defined as any unfavourable or unintended sign or symptom presented or reported by the subject regardless of its relationship with the device. Each patient was carefully monitored for adverse events, including alterations in clinical and laboratory tests considered to be clinically relevant by the investigator. The investigator questioned the patient about the presentation of adverse events and recorded those reported. All the adverse events with inconsistencies in terms of date correlation with Essure® insertion were considered as possibly unrelated to the device. Additionally, adverse events in patients receiving other treatments simultaneously were assumed to not be clearly related to only Essure®. The possible causality was determined by the investigator reviewing the clinical data of each patient.
Statistical analysis: Results were expressed as means ± standard-deviation for quantitative variables and as total numbers and percentages for qualitative variables. The data were analysed using a personal computer based software package (G-Stat 2.0. Glaxo Smith Kline, Tres Cantos. Madrid). Normal distribution and variance homogeneity were verified by the Shapiro-Wilk test, and Levene and Bartlett tests. The Student’s t test was used to compare the mean scores between groups. Intergroup differences were evaluated with ANOVA or Kruskall-Wallis according to homogeneity of variance measured with the Bartlet’s test. The Chi squared test was used for the interpretation of qualitative variables. The level of significance was established at 5% (p<0.05).
Results
The clinical characteristics of the patients studied are shown in (Table 1). Thirty-six patients (23.08%) in the study group (SG) were allergic to nickel compared to only 4.49% (n=7) in the control group (CG) (p<0.01). No differences were found in other metal allergies, years since device insertion, previous joint pain, mental disorder, fibromyalgia, previous interventions or previous deliveries.
No differences were detected between groups regarding the ease of insertion and pain during the procedure. The 156 users in the SG had had the device for a mean of 5.78 ± 0.25 years compared to 5.75 ± 0.27 in the CG (p=0.94). On comparing the difficulty in insertion between cases and controls, in 110 (70.51%) and 119 (76.28%) women, respectively, the insertion was carried out without problems (p=0.50). On the other hand, up to 8.33% (13) of women in both groups referred significant pain during insertion.
Three months after device insertion 80.12% (125) of the patients in the SG were asymptomatic while the remaining patients 19.88% (31) reported mild or moderate pain at only 1.84 ± 0.06 days after insertion. On the other hand, 78.21% (122) of the patients in the CG were asymptomatic (p=0.70) while the remaining patients reported pain for1.78 ± 0.07days (p=0.54). At this time, no differences were observed in patient satisfaction or related adverse events.
Currently, 58.61% (93) of the patients in the SG and 2.56 % (4) in the CG describe pelvic pain as being the most bothersome symptom (p<0.01). A total of 17 women (10.89%) in the SG reported abnormal uterine bleeding in the form of abundant menstrual haemorrhage compared to 4.49% (7) in the CG (p=0.03). Allergy was reported in 5.12% (8) of SG patients versus 0 (0) in the CG (p=0.02). In addition, more women in the SG had polyarthralgia and urinary tract infection (4.48% vs. 0%, respectively (p=0.03)). Moreover, some patients in the SG described additional complaints (Table 2) (Figure 1) at 15.59 ± 1.87 months after device insertion compared to 9.20 ± 5.50 months in the CG. Pain treatment included the use of NSAIDs or metamizole in 58.33% (91) and 2.56% (4) of cases in the SG and the CG respectively, and hormonal contraceptives or antifibrinolytics were prescribed to decrease bleeding in 3.2% (5) of women in the SG, whereas no treatment was required in the CG.
Among the reasons for device withdrawal, the immediacy of Essure® insertion and symptom debut was reported by 48.07% (75) of the women. Up to 10.25% (16) of the patients explained that they had not previously experienced the present complaints, and 13.46% (21) reported that the reporting of possible adverse events appearing in journals and on social media led to their claim. Likewise, 8.97% (14) requested device removal after being recommended to do so by another doctor.
In the SG, 47 patients underwent further surgery. In 97.88% (46) of the cases device removal was by laparoscopy and in one case by hysteroscopy. Complications included 4 hysterectomies due to incomplete extraction of the device (8.51%). In 1 patient, a cornual incision was needed to remove the device. Three months later, 78.72% (37) of the patients reported feeling well or very well, and 85.10% (40) reported feeling better than before the surgery (Figure 2). This improvement occurred within the first month after surgery in 87.23% (41) of cases. However, up to 93.67% (43) of the patients continued to describe some complaints (headaches, pain, alopecia) following device removal.
No relevant changes were observed in the uterus at the time of laparoscopy; however, up to 25.53% (12) of patients had peritoneal adhesions, 6.38% (3) endometriosis and in 1 patient bilateral hydrosalpinx was found. Histological examination of the uterine tube following removal of the device showed fibrosis and minimal and non-specific changes in 12.76% (6), 38.39% (18), and 38.39% (18) of the cases, respectively and 10.63% (5) showed a normal tubal histology.
Discussion
Several studies have reported that patients carrying the Essure are highly satisfied with the device [3,6]. As in the study by Chudnoff, we found that the most common symptoms are pelvic pain, irregular menstruation, dyspareunia and spotting [7]. However, while in the study by Chudnoff the patients reported the appearance of symptoms along the 5-year follow-up period, in the present study, the debut of symptoms varied greatly, with some patients describing symptoms immediately after device insertion and others reporting problems at 12 years. In addition, in the survey performed at three months, no symptoms were referred immediately after insertion [3], although at present some patients have reported that they had actually had the symptoms since the day after device insertion. These reports merit special attention since it is well known that some asymptomatic patients may start to perceive adverse events after having heard about them from other patients or on the media or social networks [8].
Pelvic pain is the main symptom described by most patients. In the study by Yunker, 7.1% of patients reported pain 3 months after device insertion which continued 6 months later in 4.2% of the cases [9]. Franchini et al. described minimal pain after insertion in 1968 women, with only 9 cases of intractable pelvic pain [10]. In contrast, in our sample, 19.88% of the women in the SG only referred mild or moderate pain for 1.84 ± .06 days after insertion. Moreover, Perkins et al. suggested that these pain rates were lower than in patients who opted fortubal ligation [11]. Likewise, in our study, pain prior to insertion and a history of fibromyalgia were found to be the most valuable predictors for long-term pain complaints [9]. In addition, in the present series, a significant number of patients in the SG group presented some type of mental illness. Therefore, in order to determine whether symptoms may be attributable to Essure®, it a necessary to obtain a comprehensive medical history to establish an adequate diagnosis and exclude other potential causes. On the other hand, a study by Perkins demonstrated that pain rates after Essure insertion were lower compared with those observed after laparoscopic tubal ligation [11].
Device removal in symptomatic patients usually correlates with an improvement in symptoms. In a recent study done by Britoin a short series of 11 patients, up to 72% of women reported an improvement after resection, while the rest remained the same [12]. In contrast, in the present series, 85.10% of the patients described an improvement suggesting that a large proportion of symptoms may not be due to the device.
A large number of patients receiving the Essure device are allergic to nickel. Previous studies have reported a low incidence of allergic patients with a risk of sensitisation after device insertion [13,14]. However, in the present study, 23.08% of the subjects in the SG were allergic to nickel versus 7.05% in the CG (p<0.01), and 14.74% and 10.26, respectively were allergic to other metals, suggesting that patients allergic to other metals may also have a nickel allergy. Therefore, nickel and metal allergies must be considered when indicating Essure® as a method of sterilisation.
Although the device can be successfully removed by hysteroscopy, the manufacturer rules out this procedure [15,16]. Therefore, in the absence of contraindication, laparoscopy is the preferred technique since salpingectomy conserves sterilisation and provides prophylax is against epithelial ovarian carcinoma [17,18].
Regarding the possibility of surgical intervention and hysterectomies, Jokinen et al. described a higher frequency of these procedures when the Fisher sterilisation technique was performed [19]. In our series, of the 47 surgical procedures carried out for device removal, 4 (8.51%) involved hysterectomies due to the inability to remove all the components of the device by salpingectomy. Patients should therefore be informed about the possibility of a subsequent hysterectomy for complete removal of the device.
Some studies have reported alterations in menstrual pattern following Essure® insertion. Bradley described that 5 years after insertion the most common complaints were irregular menstruation (14.8%), intermonth bleeding (18.8%), heavy menstrual flow (37.5%) and low menstrual flow (23.3%) [6]. Perkins highlighted the greater rate of abnormal uterine bleeding with hysteroscopy sterilisation compared to laparoscopic procedures (26.8% versus 22.3%) [10]. In the present series, most of alterations in the bleeding pattern improved after device withdrawal, although abnormal bleeding continued to be reported by 23.72% of the cases.
This study is mainly limited by its retrospective cohort case-control design and by the lack of more objective measures such Bold-fMRI, C-polymodal nociceptors activation or micro neurographic records. The survey did not ask the participants if they had been previously diagnosed with fibromyalgia or if they had presented pelvic pain preceding insertion of the device, all these data was recorded after the patient complained with pain. Other limitations include the lack of data on pathologies that may course with neuropathic pain such as diabetes or postherpetic neuralgia. Moreover, in the present study, the condition of patients carrying Essure® was assessed, showing a wide range of symptoms which partially improved upon device withdrawal being difficult to demonstrate a cause-effect relationship. Further well-designed studies, able to establish a potential causal relationship between Essure® and the above-mentioned symptoms are needed. According to Advancing Minimally Invasive Gynecology Worldwide (AAGL) [20], appropriate patient selection and careful preoperative assessment are required when considering the use of any intrauterine device. Moreover, women should be informed about the benefits and potential risks of hysteroscopy and traditional sterilisation procedures [21].
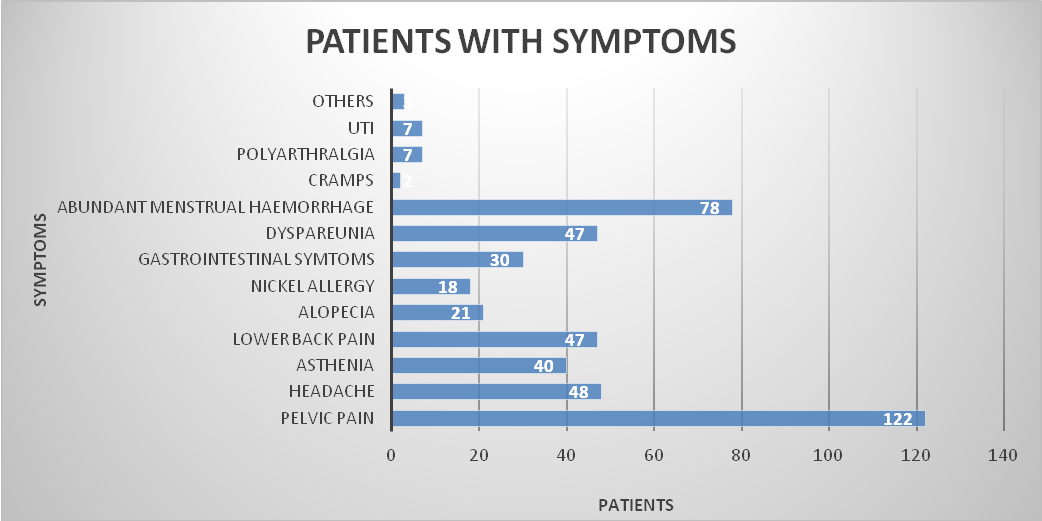
Figure 1: Symptoms reported by patients requesting Essure® removal (N=156).
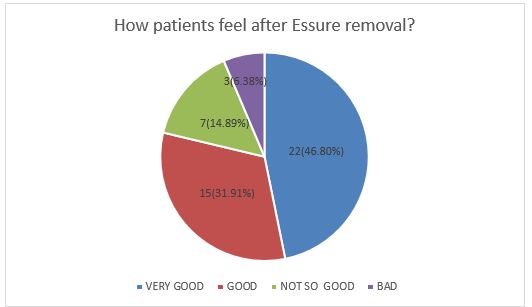
Figure 2: Degree of patient satisfaction after Essure® removal (N=47).
|
|
SG group n=156(%) |
CG group n=156(%) |
p |
|
Age (years) |
36.74 ± 0.41 |
36.38 ± 0.44 |
0.56 |
|
Years since device insertion |
5.78 ± 0.25 |
5.75 ± 0.27 |
0.94 |
|
Nickel allergy |
36 (23.08) |
7(4.49) |
<0.01 |
|
Other metal allergy |
23 (14.74) |
16(10.26) |
0.23 |
|
Previous joint pain |
8 (5.13) |
7 (4.49) |
0.79 |
|
Mental disorder |
7 (4.52) |
11 (7.05) |
0.33 |
|
Fibromyalgia |
3 (1.93) |
2(98.72) |
0.65 |
|
Previous surgery |
0.33 ± 0.04 |
0.33 ± 0.04 |
0.30 |
|
Previous deliveries |
2.21 ± 0.07 |
2.40 ± 0.08 |
0.08 |
|
Insertion without difficulty |
110 (70.51) |
119 (76.28) |
0.20 |
|
Significant pain during insertion |
13 (8.33) |
13 (8.33) |
|
|
Asymptomatic patients after 3 months |
125 (80.12) |
122 (78.21) |
0.70 |
|
Days with pain after insertion |
1.84 ± 0.06 |
1.78 ± 0.07 |
0.54 |
|
Patients score (1-10) |
9.55 ± 0.06 |
9.48 ± 0.06 |
0.47 |
|
Satisfied or very satisfied patients |
153 (98.08) |
150 (96.15) |
0.31 |
Table 1: Clinical characteristics of the patients studied. Data are given as mean ± standard deviation for quantitative values and as total numbers and percentages for qualitative values. (SG: study group; CG: control group).
|
Most bothersome symptom |
|||
|
|
SG* n=156(%) |
CG n=156 (%) |
p |
|
Pelvic pain |
93 (58.61) |
4 (2.56) |
<0.01 |
|
Headache |
5 (3.2) |
3(1.92) |
0.43 |
|
Asthenia |
6 (3.84) |
1(0.64) |
0.09 |
|
Lower back pain |
5 (3.21) |
4(2.56) |
0.73 |
|
Allergy |
8 (5.12) |
0(0) |
0.01 |
|
Gastrointestinal symptoms |
2(1.28) |
1(0.64) |
0.56 |
|
Dyspareunia |
1(0.64) |
1(0.64) |
|
|
Abundant menstrual haemorrhage |
17 (10.89) |
7 (4.49) |
0.03 |
|
Cramps |
2 (1.28) |
0(0) |
0.27 |
|
Polyarthralgia |
7 (4.48) |
0(0) |
0.03 |
|
Urinary tract infection |
7 (4.48) |
0(0) |
0.03 |
|
Others |
3 (1.92) |
1(0.64) |
0.31 |
Table 2: The most bothersome symptoms reported by the patients included in the study. Data are given as total numbers and as percentages in brackets. *SG: study group; CG: control group.
Citation: Castelo-Branco C, Duro-Gomez J, Romero-Dominguez M, Rodriguez-Marin AB, Povedano-Canizares B, et al. (2019) Essure-Attributed Symptoms: Follow-up of a Large Cohort after Device Removal: A Retrospective Nested Case-Control Study. J Obstet Gynecol Probl: JOGP-100002.