Journal of Surgery and Insights (ISSN 2652-4643)
Volume 04; Issue 01
Review Article
An Interventional Echocardiographic Screening Program for First Degree Relatives of Patients with Bicuspid Aortic Valve: Is It Practical?
Chan Siang Kan*1, Nurul Hafizah Zailani1, Rafandi Zuki2, Bujang MA3, Sing Yang Soon1, Yuan Hsun Jong1
1Department of Cardiothoracic Surgery, Sarawak Heart Center, Kota Samarahan, Sarawak, Malaysia.
2Department of Cardiology, Sarawak Heart Center, Kota Samarahan, Sarawak, Malaysia.
3Clinical Research Center, Sarawak General Hospital, Sarawak, Malaysia.
*Corresponding author: Chan Siang Kan, Department of Cardiothoracic Surgery, Sarawak Heart Center, Kota Samarahan, Sarawak, Malaysia, Tel: + 60167689015
Citation: Kan CS, Zailani NH, Zuki R, Bujang MA, Soon SY, Hsun Jong Y (2022) An Interventional Echocardiographic Screening Program for First-Degree Relatives of Patients with Bicuspid Aortic Valve: Is It Practical. J Surg Insights: JSI-100036
Received date: 04-April,2022; Accepted date: 11-April,2022; Published date: 19-April,2022
ABSTRACT
Bicuspid Aortic Valve (BAV) represents one of the most common congenital cardiac anomalies with a prevalence of 0.5-2% in the general population. Literature demonstrated that the prevalence of BAV among First-Degree Relatives (FDR) of those with diagnosed BAV ranges from 7.3% to 9.1%. BAV has diverse genetic etiologies that vary from complex inheritance in families to sporadic cases without any evidence of inheritance.
This interventional study aims to identify potential subjects with BAV by screening the FDRs of patients with BAV, which allows early detection of aortic valvular dysfunction and aortic complications associated with BAV. In addition, we hope to explore the practicality of running a routine ECHO screening program for FDR of patients with BAV in a tertiary hospital setting by analyzing the outcomes.
We identified a total of 51 patients who had undergone aortic valve surgery performed at Sarawak Heart Centre (previously Sarawak General Hospital) in the context of BAV disease from p 2002 to 2018. This study only recruited the FDR of patients with true bicuspid aortic valves. Interviews with BAV patients and their family members were conducted to obtain a detailed minimum three-generation family history. Standardized, complete two-dimensional and Doppler transthoracic echocardiograms (ECHO) were performed on all participants by a single expert echocardiographer with more than 20 years of experience.
Results
Fifty-one subjects with a mean age of 37.4 (±14.9) years were recruited. Majority were females [27 (52.9%)], Chinese [35 (68%)] and non-smokers [44 (86.3%)]. The prevalence of newly diagnosed BAV in our cohort of FDR was 7.8% (4 out of 51 FDR). The average AV Max Velocity in the BAV group was higher than the TAV group by a mere 0.04 m/sec, and the difference was statistically significant [1.66 (±0.97) m/sec vs. 1.26 (±0.30) m/sec respectively, p=0.05]. Only 1 out of the four patients diagnosed with BAV in this screening program demonstrated mild aortic stenosis (AV Max Velocity = 3.1 m/sec and AV mean PG = 10 mmHg). Two out of the four patients with newly diagnosed BAV came from a family of the same proband or indexed patient. 2 out of 51 patients (without BAV) screened had demonstrated aortic root dilatation (aortic root diameter of more than 4.0 cm).
Conclusion
The prevalence of BAV is higher in FDR of BAV patients than in the general population. Therefore, echocardiographic screening of FDR is a practical, low-cost approach for identifying asymptomatic cases to allow intervention before complications arise. A routine ECHO screening program should be considered at the institutional level or even better at the national level. Future research on this screening program's long-term practicality and cost-effectiveness is needed to produce a well-structured and systematic ECHO screening program and validate the long-term benefit.
INTRODUCTION
Prevalence
A standard aortic valve possesses three leaflets - left, right, and non-coronary cusps. Bicuspid aortic valve (BAV) is a condition where, instead of typical three leaflets aortic valve in the normal heart, only two unequal-sized leaflets are present and indirectly create flow disturbance. A small percentage of malfunctioning congenitally abnormal aortic valves are unicuspid or possess a single commissure [1]. BAV is one of the most common congenital cardiac anomalies, with a prevalence of 0.5-2% in the general population [2]. In the general population, BAV is more prevalent in Men (1-2%) than women (0.5%) at a ratio of ~3:1 [3,4]. Approximately 3:1 suggesting the loss of genes on the X chromosome may predispose to BAV syndrome [5].
BAV is one of the most common congenital cardiac anomalies, prevalence 0.5-2% in general population.2 In general population, BAV is more prevalent in Men (1-2%) than women (0.5%) at a ratio of ~3:1 suggesting the loss of genes on the X chromosome may predispose to BAV syndrome[3-5]. Carro A et al. demonstrated that the prevalence of BAV among the FDR screened was 7.3%, significantly higher than that reported in the general populations (0.5-1.0%) [6]. Generally, the prevalence of BAV among first-degree relatives (FDRs) of affected individuals is 9% to 21% [7,8]. Whereas Siu SC et al. found out that the prevalence of BAV stands nearly 10-fold higher in primary relatives of patients with BAV than in the general population [5]. FDR's chances of getting BAV in a family with 2 or more affected members was reported to be even higher at 28-37%. In contrast to families with inherited BAV disease, more than 80% of patients with BAV have no known affected relatives and are regarded as sporadic cases [9].
Anatomy & Pathology
In the early pathology studies, there were three crucial characteristics of BAV: inequality of cusp size, presence of central raphe or ridge in the center of the larger of the two cusps, and smooth cusp margins even in the diseased valves [10]. When a tricuspid aortic valve (TAV) has two cusps become fused due to rheumatic or other inflammatory processes, this is also called pseudobicuspid [11]. Post-inflammatory valves, cusp margins tend to be severely distorted and fused, resulting in the valve often having a central tri-radiate orifice. Some confusion may still be present. When doubt exists, histopathological examination (HPE) can help to distinguish between acquired or congenital BAV. The latter shows no valve tissue in the raphe, whereas post-inflammatory valves show evidence of previous valvitis [11,12]. Stenosis usually develops in BAV containing no redundant cusp tissue, and incompetence often in valves in which redundancy and prolapse are prominent [13]. In cases of BAV, the ordinarily thin aortic valve cusps often prematurely calcify, leading to valvular thickening and stenosis [14].
Genetic
BAV has highly variable phenotypic characteristics and diverse genetic etiologies ranging from complex inheritance in families to sporadic cases with no evidence of inheritance. Some subjects who transmit BAV to their offspring who are assumed to harbor causative genetic mutations may not manifest BAV clinically themselves or have other cardiovascular abnormalities. Linda Cripe et al. demonstrated high heritability in 50 probands with BAV, suggesting that the BAV determination is almost entirely genetic (Heritability (h2) of BAV and BAV +/- cardiovascular malformation were 89% and 75%, respectively) [15]. Growing evidence supports its familial predisposition with an autosomal dominant pattern of inheritance that occurs without syndromic features [2,17]. The inheritance pattern of BAV may also be autosomal dominant with incomplete penetrance or a Mendelian inheritance pattern [5,18]. By genome-wide scan of the available family members with polymorphic microsatellite markers and linkage analysis, BAV-susceptibility loci have been mapped on chromosomes 9q34-35, [19,20] 18q, 5q15-21 and 13q33-qter, and NOTCH1 (which is involved in the initial stages of valve formation involve multiple signaling molecules) [21]. Of these, the mutation in NOTCH1, a single-pass transmembrane receptor that functions in a highly-conserved pathway, plays critical roles in cell fate determination during organogenesis, were associated strongly with non-syndromic BAV in humans [19,23]. However, genetic testing for NOTCH1 mutations remains unclear for non-familial cases.
Classification of BAV
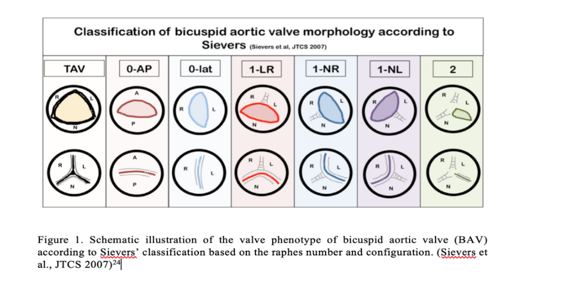
There are different variants of BAV morphology. Morphology determines the natural history, clinical presentation, and long-term prognosis. The “purely” BAV is composed of two cusps, morphologically and functionally [24]. Classification of BAV according to Schaefer: Type I Fusion between RCC and LCC (R-L) occurs most frequently (70%), followed by type II fusion of RCC and NCC (R-NC) (28%). LCC and NCC (Type III fusion) were rarely fused (1.4-2%) [25,26]. Type I (R-L) fusion is associated with additional congenital heart disease/cardiac malformation, for example, coarctation of the aorta (CoA), interrupted aortic arch, and hypoplastic left heart syndrome[5,25]. Type II (R-NC) cusp fusion is associated with atrioventricular valve (AV) dysfunction/ cuspal pathology [aortic regurgitation (AR) or aortic stenosis (AS)] and minor association with aortic root dilatation [25, 27]. Sievers et al. also classified BAV into three significant types were identified: type 0 (no raphe), type 1 (one raphe), and type 2 (two raphes) (Figure 1). Shows the schematic illustration of the valve phenotype according to Sievers’ classification based on the raphes number and configuration. Type 0 has a 6% prevalence; type 1 is the most common phenotype with a prevalence of 89%, whereas type II has a prevalence of 5%. Type 0 can further be classified into 0-AP (anteroposterior) or 0-lat (Lateral) based on the configuration of cusps. In addition, type 1 is further categorized into subtypes: 1-LR for the fusion of the right and left coronary cusps (71%), 1-RN for the fusion of the right, and noncoronary cusps (15%) and 1-NL for the fusion of the noncoronary and left coronary cusps [24].
Association of BAV to Valvular dysfunction & Vascular Anomalies
BAV is a clinically heterogeneous disease with a high incidence (up to 35%) of surgery-related complications of the aortic valve and ascending aorta. The morbidity of BAV is extremely high already in younger adulthood, and 25% of affected people experience in the course of their life would develop severe aortic valvular dysfunction, ascending aortic aneurysm, cardiac mortality, hospital admission for heart failure, and aortic dissection or rupture [28]. A BAV occurs in 20-85% of cases of CoA [29-32]. The presence of an untreated, inadequately treated, or recurrent coarctation increases the likelihood of developing aortic stenosis, aortic regurgitation, or dissection of the aorta. BAV has also been reported in 27% of 52 cases of interrupted aortic arch suggesting a common developmental pathogenesis [33]. BAV accounts for 70-85% of pediatric patients' aortic stenosis (AS) and at least 50% of the stenotic aortic valve in adults [7,31]. Severe AS (1.5-71%) in BAV are very rigid because of fibrosis and heavy calcification but are not narrowed [12,35]. Aortic regurgitation (AR) (1.5-40%) is more complex than AS. The etiology of AR in patients with BAV usually results from prolapse of larger unequally sized cusps [13], an association of aortic dilataion [36], coarctation of the aorta, or infective endocarditis [10]. Disruption or dissolution of elastic tissue within the upper aortic ring (STJ) secondary to root dilatation leads to AR (50%), as this structure provides the main support for the valve cusps [36,37]. An autopsy study in adults suggested that about 30% of congenitally bicuspid aortic valves become stenotic, 40% become regurgitant, and the remainder function normally[34]. A large pathological review by Sabet and colleagues revealed that BAV disease results in a stenotic lesion in three-quarters of patients, insufficiency in 15%, and a mixed lesion in 10% [38].
Calcification and fibrosis are age-related BAV cusps that often prematurely calcify, leading to valvular thickening and stenosis [35]. Stenosis is rapid if the aortic cusps are asymmetrical or in the anteroposterior position [13]. Sclerosis of valve begins in the second decade whereas calcification is increasingly prominent from fourth decade onwards. Calcification in BAV is progressive and more diffuse than degenerative aortic valve disease [39]. In addition, patients with poor lipid profiles and those who smoke are also at an elevated risk of developing hemodynamically significant bicuspid aortic stenosis [40]. These are potentially modifiable risk factors amenable to treatment. Approximately half of the young adults with a BAV have aortic root dilatation [5,41]. Thus, potential candidates for resultant aortic regurgitation. Aortic root dilatation, a precursor of dissection, occurs in 50-60% of patients with a normally functioning BAV [41,42]. When the aortic root reaches 6cm, the aortic dissection risk increases 9-fold [43]. Average annual changes in ascending aorta in patients with BAV vary between 0.2 to 1.2 mm/year [44-48]. Several authors have suggested that ascending aortic dilation is one component of the bicuspid syndrome inherited together with BAV. However, both conditions do not necessarily appear together in the same individual.
BAV is present in 1-13% of unselected cases of aortic dissection [13]. The presence of a BAV increases the risk of dissection 9-fold; this rises to 18-fold if there is a unicommissural aortic valve [43]. The actual incidence of aortic dissection is debated. Although the prevalence varies depending on the cohort studied, a pooled estimate of cases of dissection associated with BAV was 4% [49,50]. The reason for the high incidence of aortic dissection in BAV is unclear. The life-threatening complication of aortic dissection in the setting of BAV is rare in childhood or adolescence. Several authors have proposed ascending aortic dilation is one component of the bicuspid syndrome inherited together with BAV. The Tricuspid aortic valve usually has right coronary artery (RCA) dominance, and the left main stem averages 10 mm in length. With a BAV, left dominance is more common (29.0-56.8%), and in 90% of cases, the left main stem is less than 5mm in length [51]. It is vital to recognize that these associations may result in inadequate myocardial perfusion.
Diagnosis
In childhood, BAV disease is commonly asymptomatic. It is estimated that only 1 in 50 children have clinically significant valve disease by adolescence [52]. During clinical examination, the presence of an ejection click with or without an ejection systolic murmur over the aortic area lacks predictive accuracy as it may be present in tricuspid aortic stenosis [13]. Auscultatory findings include an ejection sound best heard at the apex. There may be associated murmurs of aortic stenosis, incompetence, or coarctation of the aorta when these lesions are present [5]. A single transthoracic echocardiogram (TTE) can reliably identify BAV and dilated aorta in most cases, with cardiac magnetic resonance imaging (MRI) able to define the anatomy when TTE imaging is suboptimal. In the current era, transthoracic echocardiograms usually confirm the diagnosis with sensitivities and specificities of 92% and 96% are reported for detecting BAV anatomy [5]. The positive predictive value (PPV) of a cross-sectional and Doppler echocardiography was found to be 93%.13 It can be difficult in patients with heavily calcified valves [53]. A meta-analysis by Hillebrand M et al. identified that TTE has a pooled sensitivity of 87.8% and a pooled specificity of 88.3% for BAV. This study showed that TTE yields almost ideal diagnostic accuracy when ideal investigators examine ideal patients [54]. Echocardiographic diagnosis demonstrates two cusps and two commissures during short-axis view. Other supportive features include cusp redundancy, eccentric valve closure, single coaptation line between cusps during diastole (closure of the aortic valve). The predictors of an inaccurate diagnosis of BAV include TTE in non-tertiary care settings, concomitant aortic aneurysm, and presence of severe aortic valve calcification [54]. A prominent raphe that can give the appearance of a third coaptation line produces a false-negative result. In contrast, a false-positive may be produced when one coaptation line of a TAV is unclear. Differentiating severe bicuspid aortic stenosis from severe unicuspid unicommissural aortic stenosis can also be difficult, but this is particularly vital when considering aortic valvuloplasty. To establish the diagnosis, the valve must be visualized in systole in the short-axis view. During diastole, the raphe can make the valve appear trileaflet [5]. Patient-related factors such as patients with obesity, chest wall deformities, narrow intercostal spaces, and the presence of pulmonary emphysema can produce suboptimal imaging quality during TTE [54]. Suboptimal imaging quality may also result from preventable investigator-related factors. These include suboptimal positioning of patients, suboptimal angulation of ultrasound probe, incomplete assessment of all aortic valve cusp structures, incomplete use of all available views, imaging modalities, and software options [54].
Importance of Family Screening
As BAV and TAA are asymptomatic and exhibit only a few signs (if any) until either hemodynamical changes or aortic dissection occurs, earlier detection must be of vital importance. Eventually, a significant percentage of patients with BAV require cardiac intervention during their lifetimes, up to 40% during the fifth decade [55]. By definition, a screening Program is worth when the disease screened is asymptomatic and not readily identified during routine care, a screening test is available that can reliably identify the disease, an effective treatment is available for the disease, and lastly, early intervention can alter the outcome of the disease [56]. Because of familial clustering and aggregation of isolated BAV or BAV with vascular anomalies [17]. it might be appropriate to screen FDR. FDRs of BAV patients should be screened for the presence of BAV and dilation of the ascending aorta due to its significant association as FDR are also at risk of developing aortic complications without BAV [57]. Because many of these BAV-related complications can be predicted or prevented, the identification of BAV heritability supports the previous recommendation that echocardiographic screening of first-degree relatives of patients with BAV is warranted in order to identify persons with structural cardiac abnormalities [17]. (Table 1) summarizes the guidelines on family screening recommendations and surgical intervention for bicuspid aortic valve patients/bicuspid aortic-related aortopathy published for the last decade [58-65]. Despite well-established clinical guidelines for screening BAV and defining the timing of operative interventions to prevent complications outlined by WHO, family screening is still not widely practiced worldwide.
OBJECTIVE
This study aims to explore the practicalities of running a routine echocardiographic screening program for FDR of patients with surgically diagnosed BAV. With a simple TTE, we sought to determine the prevalence of BAV in FDRs of patients with isolated BAV and the occurrence of aortic dilatation/familial BAV. This screening program will permit an early diagnosis of the valve and aortic anomaly in family members of BAV patients. Early detection of the problem allows intervention to occur before complications arise.
Study Design & Method
We identified a total of 55 patients with surgically confirmed BAV who had undergone aortic valve surgery performed at Sarawak Heart Center (previously Sarawak General Hospital until the year 2010) from the year 2002 to 2018. In all cases, the aortic valve morphology and the etiology of aortic stenosis/regurgitation were determined by surgical inspection of the valve intra-operatively. Therefore, this study only considers the true bicuspid aortic valves diagnosed during surgery rather than an echocardiogram. We excluded patients with an aortic valve that exhibited two cusps but three sinuses with three interleaflet triangles indicating an acquired rather than the congenital bicuspid structure of the aortic valve (pseudobicuspid aortic valve). Out of the 55 patients identified, 15 of them died at the time of recruitment. In the remaining 40 patients, 19 were uncontactable for various reasons. Interviews with the patients were conducted by telephone. Finally, nine patients and their family members were agreeable to being recruited into this interventional project. Among the nine families, the member recruited for each family ranged from one to fifteen family members per family. The total number of first-degree relatives screened was 51 subjects. We obtained a detailed minimum three-generation family history via interviews with BAV patients and their family members. Of which, patient or their families expressing interest in the study were contacted by phone or during outpatient visits to request participation. Each proband’s FDR willing to participate were enrolled into the study. For every newly affected individual identified, all of that individual’s FDR was subsequently evaluated (Sequential Sampling). Sampling was extended to Second-degree relatives when medical history or study echocardiography identified additional affected individuals.
Detailed physical examinations were performed on each subject. Anthropometric measurements (height and weight) were taken, and body mass index was calculated in all subjects. Vital signs during the assessment were recorded and analyzed. In addition, thorough cardiovascular examinations were carried out to identify the presence of systolic murmur and other signs that may suggest valvular dysfunction and related aortopathy. Standardized, complete two-dimensional, and Doppler transthoracic echocardiograms were obtained on all participants. A single expert echocardiographer of more than 20 years’ experience was allocated to perform and interpret all echocardiograms. It was then followed by detailed validation by a second expert echocardiographer. ECHO assessment includes aortic valve leaflets and morphology (assessed in both systole and diastole), other valves structures, AV Max Velocity, AV Mean PG, EF, and BAV Phenotypes (AP Configuration, Right-Left Configuration). Other supportive structures of BAV include cusp redundancy, valve thickening, and eccentric valve leaflet closure. Individuals who had aortic valves with two clearly defined cusps or with the characteristic systolic fish mouth appearance of the aortic valve cusps and 2 of 3 supportive features of BAV, including systolic doming or diastolic prolapse of the aortic valve cusps and eccentric valve leaflet closure, were considered to have a BAV. The diameter of the aortic root dimension was measured at end-diastolic in the parasternal long-axis view, leading edge to leading edge. Sinus of Valsalva and tubular ascending aorta was dilated if the diameter exceeded 40 mm.
Ethic Approval
This study complied with the ethical principles outlined in the Declaration of Helsinki and the Malaysian Good Clinical Practice Guideline. This research was registered under the National Institute of Health, Ministry of Health, Malaysia via the National Medical Research Registry (NMRR Number: NMRR-19-2429-46869).
Consent
Written informed consent and a complete medical history were obtained from all participants during the first interview session.
Statistical Analysis
Descriptive statistics were used to summarize the demographic characteristics of the participants according to the group. Data were reported as mean ± standard deviation and median (minimum-maximum range) for ordinal or continuous variables and as numbers and percentages for categorical variables. The mean of continuous and categorical variables was compared using the One-way ANOVA test analysis. P values < 0.05 were considered statistically significant. All analyses were performed using Statistical Package for the Social Sciences (SPSS) version 23.
RESULT
Fifty-five patients with surgically diagnosed BAV underwent surgical aortic valve replacement (AVR), or AVR with concomitant Aortic surgery were identified from an institutional database. After a thorough screening of the patients, excluding those who had passed away, lost to follow-up, uncontactable by phone, and those who refused to participate in the screening program, only nine patients and their family members, were agreeable to be recruited into this interventional project. Among the nine families, the member recruited for each family ranged from one to fifteen individuals per family. The total number of first-degree relatives recruited and screened was 51 subjects. (Average 5 FDR per indexed patient) The age of the subjects screened ranges from 7 to 66 years old with a mean age of 37.4 (±14.9) years, and an almost equal number of males and females [24 (47.1%) and 27 (52.9%) respectively]. Sixty-eight percent (n=35) of the subjects were Chinese, followed by 17.6% of Malay (n=9) and, lastly, 13.7% of local Sarawak ethnic known as Iban (n=13.7%). The subjects had an average body mass index of 25.2 (±6.9) Kg/m2, and the majority of them were non-smokers (n=44, 86.3%). (Table 2)
As many as 17 of them (33.3%) had some concurrent medical illnesses such as hypertension (n=11, 21.6%), type 2 diabetes mellitus (n=2, 3.9%), ischaemic heart disease (n=2, 3.9%), obstructive sleep apnea (n=1, 2%) as well as bronchial asthma (n=1, 2%). The common cardiovascular presentations BAV and its related complication in this population were consists of presyncope attack (n=5, 9.8%), angina pectoris (n=1, 2%) and reduction in effort tolerance (n=5, 9.8%). Most of the clinical symptoms above were present in patients later diagnosed with at least one valvular pathology. During the screening, an assessment of vital signs was performed after the patient went through an interview session. Eighteen subjects had a systolic pressure of more than 140 mmHg during the assessment; the range of systolic and diastolic blood pressure was between 81 to 170 mmHg [Mean of 133 (±23) mmHg] and 52 to 108 mmHg [Mean of 81 (±12) mmHg] respectively. All subjects who participated in this screening program had regular pulse rate [range 50 to 95 beats per minute, mean 74 (±12)] and respiratory rate during the assessment. Two patients were found to have systolic murmur during the cardiovascular examination. (Table 3)
The prevalence of newly diagnosed BAV in our cohort of FDR was 7.8% (4 out of 51 FDR). Two out of the four patients with newly diagnosed BAV were coming from a family of the same proband or indexed patient. Including the BAV, the screening program had identified seven subjects (13.7%) with abnormal aortic valve morphology such as BAV, quadricuspid aortic valve, thickened RCC, and NCC. One of the four patients with true BAV had started to show a significant degree of aortic stenosis. Two patients with tricuspid aortic valves also presented significant aortic regurgitation during the echocardiographic screening. Regarding aortic pathology, 2 out of 51 patients screened had demonstrated aortic root dilatation (aortic root diameter of more than 4.0 cm). On top of the aortic pathology and aortic valvular abnormality detected, this echocardiographic screening program also incidentally picked up seven subjects (13.7%) with at least moderate mitral regurgitation and two subjects (3.9%) with significant tricuspid regurgitation. (Table 4)
(Table 5) compares echocardiographic parameters between the subjects with BAV and TAV. The mean left ventricular ejection fraction (LV EF) of both groups appears to have no significant difference and was both normal - higher than 55% [63.3 (±5.92) % vs. 64.3 (±5.73) % respectively, p=0.732]. Three important parameters to assess the severity of aortic stenosis, particularly in the presence of BAV; aortic valve maximal velocity [AV Max Velocity (measured in meters/seconds or m/sec)], aortic valve mean pressure gradient [AV mean PG (measured in mmHg)] and aortic valve area [AVA (measured in cm2)]. The average AV Max Velocity in the BAV group was higher than the TAV group by a mere 0.04 m/sec, and the difference was statistically significant [1.66 (±0.97) m/sec vs. 1.26 (±0.30) m/sec respectively, p=0.05]. This result appears to be similar for AV mean PG, where the mean PG across aortic valve in the BAV group was higher than that of the TAV group; however, the difference was not significant. [5.00 (±3.34) vs 3.67 (±1.35) respectively, p=0.107]. Only 1 out of the four patients diagnosed with BAV in this screening program demonstrated mild aortic stenosis (AV Max Velocity = 3.1 m/sec and AV mean PG = 10 mmHg). Both groups' mean aortic valve area showed little difference, although BAV had slightly smaller AVA than TAV. [BAV, 2.23 (±0.84) cm2 vs TAV, 2.41 (±0.62) cm2, p=0.571]. The average aortic root diameter for the subjects was 26 (±6.10) mm for BAV and 26.7 (±3.65) mm for TAV groups, and there was no difference (p=0.770). This study identified two patients (2/51, 3.9%) with an aortic root diameter of > 40 mm without BAV.
DISCUSSION
The prevalence of newly diagnosed BAV through family screening programs in our cohort was comparable to that of other studies (7.8% in our study compared to 9% to 21%) [7-8]. Aortic dilation prevalence in FDR with TAV was relatively lower than the figures found in the literature (3.9%). Those aortic dilations were mild and more frequently observed in patients with hypertension. BAV is associated with several long-term health risks such as progressive valvular dysfunction and associated aortopathy if not identified early and managed appropriately. Therefore, it is paramount to identify at-risk family members and facilitate family screening to reduce long-term morbidity and mortality. Over the last decade, various guidelines have been published and revised on the recommendation of family screening and surgical intervention for bicuspid aortic valve patients/bicuspid aortic-related aortopathy [58-65].
Nishimura et al. suggested that young patients (<30-year-old) with AVmeanPG > 30 mmHg should have annual ECHO, and those < 30 mmHg should have every other year ECHO. Those with Ascending aortic diameter > 40 mm regardless of age, should have baseline CT or MRI for adequate visualization and annual ECHO surveillance [61]. According to the AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease published in 2016, surgical Intervention is advised for asymptomatic Ascending Aortic diameter >55mm. In contrast, the cut-off for Intervention was reduced to a diameter of 45 mm with severe AV stenosis and severe aortic insufficiency or positive family history of aortic dissection or aneurysmal rupture. Progressive aortic root dilatation > 5mm per year was also an indication for surgery. Additionally, women of childbearing age with BAV and aortic diameter > 45 mm should be advised against pregnancy, and athletes with aortic diameters > 45 mm should refrain from high-intensity sports [64]. Two years later, Borger MA et al. published an executive summary of the American Association for Thoracic Surgery consensus guidelines, the latest guideline on bicuspid aortic valve-related aortopathy.
Alice et al. conducted a study to examine the utility and cost of echocardiography screening of siblings of patients with BAV in clinical practice. They concluded that echo screening among siblings of those with BAV is effective and inexpensive. Therefore, it should be incorporated into clinical care [66]. Screening of FDR followed by serial regular surveillance imaging of individuals with identified BAV from the screening will help to prepare them for the complication that may arise in the future. In addition, the family members screened can also receive interventions to reduce cardiovascular risk factors and perhaps, emerging therapies targeted to prevent these complications. All patients should be advised to quit smoking and have well-controlled blood pressure. A beta-blocker is suitable as first-line anti-hypertensive because of its noted wall shear-stress reduction effect. Be careful in cases of severe aortic stenosis, which may reduce coronary perfusion if diastolic blood pressure is too low. Angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) are suitable for afterload reduction [67]. No additional medical treatment was needed to be initiated in any of the subjects screened. Although both the 2010 ACC/AHA guideline and 2014 ESC Guideline on Aortic Diseases recommended the prescription of beta-blockade, stringent control of hypertension, with an angiotensin receptor blocker or an angiotensin-converting enzyme inhibitor as an alternative, this remains debatable [58,62]. Given the prevalence of BAV in the general population, the recommendations for health supervision and family screening should not fall only under the purview and responsibility of the cardiologist or cardiac surgeons. Primary care and family medicine physicians should have a working knowledge of the health risks associated with BAV and its complications and the relevant screening recommendations for affected individuals and at-risk family members. However, when should cardiac screening for BAV and aortic dilatation start and at what intervals after the initial assessment repeat echocardiographic screening should occur remains unknown. Therefore, two subjects with aortic root dilatation (aortic root diameter of more than 4 cm) will undergo yearly CT surveillance. In addition, seven patients with significant MR and two patients with TR will undergo annual ECHO surveillance to monitor disease progression. The impact of this study may not be substantial, but family screening could be worthwhile. Most individuals screened are asymptomatic and will otherwise not visit a physician until the first symptoms of valvular dysfunction or aortic disease develop. Relatively inexpensive echocardiography could reliably identify the disease and prepare the individuals at risk for early detection, early diagnosis, and early intervention. However, there is uncertainty about the outcome in preventing aortic dissections. Not to forget the negative impact on patients' quality of life, stress, and cost of personal health insurance, which will be affected. Because BAV formation and disease involve many genes, developing clinical genetic tests to identify patients at risk for BAV-related complications, such as aortic stenosis or TAAD, will require considerable time and research9. Therefore, early identification and careful surveillance of at-risk family members will likely remain primary clinical strategies until then.
Limitations
Limitation from this study includes small sample size. Sarawak, a state located in East-Malaysia Borneo island, is a huge state with less accessibility to the rural area. This has becoming a challenge for the participants to come for screening due to the travelling time and cost. The rise of COVID-19 pandemic also influenced the participation rate of this study. 2 positive results represent 5% incidence from the 41 participants. At least 232 subjects are required to achieve 95% confidence interval thus reflecting a larger population.
CONCLUSION
Both local and international data suggest a high prevalence and incidence of BAV among FDR patients with this common congenital cardiac abnormality. Based on these data, the authors strongly agree with the recommendation for an echocardiographic evaluation of all FDR of patients with BAV as class IIa in search of aortic dilation and BAV. The ultimate Endpoint – reduce the potential morbidity and mortality associated with BAV and its related aortopathy. A routine ECHO screening program should be considered at the institutional level or even better at the national level. In addition, all patients with BAV should be made aware of its familial pattern of inheritance. Finally, future research on this screening program's long-term practicality and cost-effectiveness is needed to produce a well-structured and systematic ECHO screening program and validate the long-term benefit.
Authors’ Contributions
C.S.K. was the principal investigator who did the literature search and wrote the report. N.H.Z. conducted the interviews and assessments. R.Z. performed all the screening echocardiography. B.M.A. assisted in data analysis. S.Y.S. and Y.H.J. both supervised the project.
Conflict of Interest
The authors declare that they have no financial conflict of interest concerning the content of this report.
Funding Sources
None
|
Guideline |
Family Screening Recommendations |
Ascending Aorta Threshold for Surgery |
|
ACC/AHA Thoracic Aortic Disease, 2010 [58] |
First-degree relatives of patients with a bicuspid aortic valve, premature onset of thoracic aortic disease with minimal risk factors, and/or a familial form of thoracic aortic aneurysm and dissection |
>55 mm |
|
European Society of Cardiology Valvular Heart Disease Guidelines, 2012 [59] |
Screening of first-degree relatives of patients with bicuspid aortic valve with aortic root disease should be considered |
55 mm (without aortic valve dysfunction) 45 mm (with aortic valve dysfunction) |
|
Society of Thoracic Surgeons Clinical Practice Guidelines, 2013 [60] |
First-degree relatives of patients with bicuspid aortic valve should undergo imaging of the aorta |
> 50 mm |
|
AHA/ACC Valvular Heart Disease Guidelines, 2014 [61] |
NA |
>55 mm >50 mm in patients with BAV and family history of aortic dissection or progressive aortic expansion of > 5 mm per year >45 mm (with aortic valve dysfunction) |
|
European Society of Cardiology Aortic Disease Guidelines, 2014 [62] |
Screening of first-degree relatives of patients with bicuspid aortic valve may be considered |
> 50 mm with risk factors |
|
Canadian Cardiovascular Society Guidelines, 2014 [63] |
Clinical screening and imaging screening are recommended |
50-55 mm |
|
ACC/AHA Surgery for Aortic Dilatation in Patients with BAV, 2016 [64] |
NA |
>55 mm >50 mm in patients with BAV and family history of aortic dissection or progressive aortic expansion of > 5 mm per year |
|
The American Association for Thoracic Surgery (AATS) Consensus Guidelines, 2018 [65] |
First-degree relatives of patients with BAV should undergo screening echocardiography |
≥55 mm (without risk factors) ≥50 mm (with risk factors*) ≥45 mm (inpatients requiring concomitant cardiac surgery) |
|
ACC, American College of Cardiology; AHA, American Heart Association; NA, not available *root phenotype or predominant aortic insufficiency, uncontrolled hypertension, family history of aortic dissection/sudden death, or aortic growth >3 mm per year
|
||
Table 1: Guidelines on Family Screening Recommendations and Surgical Intervention for Bicuspid Aortic Valve Patients/Bicuspid Aortic-related Aortopathy.
|
Characteristics, n = 51 |
Number (%) |
Mean (±SD) |
|
Age (years) |
|
|
|
|
Range 7 - 66 |
37.4 (±14.9) |
|
Gender |
|
|
|
Male |
24 (47.1%) |
|
|
Female |
27 (52.9%) |
|
|
Race |
|
|
|
Chinese |
35 (68.6%) |
|
|
Malay |
9 (17.6%) |
|
|
Iban |
7 (13.7%) |
|
|
Body Mass Index (Kg/m2) |
|
25.2 (±6.9) |
|
Smoking History |
|
|
|
Active Smoker |
6 (11.7%) |
|
|
Ex-Smoker |
1 (2.0%) |
|
|
Non-Smoker |
44 (86.3%) |
|
|
*BAV, bicuspid aortic valve. FDR, first-degree relative. |
||
Table 2: Demographic Characteristics of FDR of patients with BAV screened.
|
Parameters, n = 51 |
Incidence (%) |
Mean (±SD)
|
|
Comorbid |
|
|
|
Hypertension |
11 (21.6%) |
|
|
Diabetes mellitus |
2 (3.9%) |
|
|
Ischaemic heart disease |
2 (3.9%) |
|
|
Obstructive sleep apnea |
1 (2.0%) |
|
|
Bronchial asthma |
1 (2.0%) |
|
|
Symptoms |
|
|
|
Syncope |
0 (0.0%) |
|
|
Pre-syncope |
5 (9.8%) |
|
|
Angina |
1 (2.0%) |
|
|
Reduced effort tolerance |
5 (9.8%) |
|
|
Orthopnea |
0 (0.0%) |
|
|
Paroxysmal nocturnal dyspnea |
0 (0.0%) |
|
|
Vital signs (at assessment) |
Range |
|
|
Systolic blood pressure (mmHg) |
81 - 170 |
133 (±23) |
|
Diastolic blood pressure (mmHg) |
52 - 108 |
81 (±12) |
|
Pulse rate (beats/min) |
50 - 95 |
74 (±12) |
|
Respiratory rate (breaths/min) |
12 - 20 |
15 (±3) |
|
CVS Examination |
|
|
|
Systolic murmur |
2 (3.9%) |
|
|
BAV, bicuspid aortic valve.; FDR, first-degree relative; CVS, cardiovascular system |
||
Table 3: Medical history and clinical presentation of FDR of patients with BAV screened.
|
Condition |
Number of affected FDR/ total FDR screened |
Incidence (%)
|
|
Bicuspid aortic valve |
4/51 |
7.8 |
|
Abnormal aortic valve morphology# |
7/51 |
13.7 |
|
Aortic stenosis* |
1/51 |
2.0 |
|
Aortic regurgitation' |
2/51 |
3.9 |
|
Aortic dilatation (>4 cm) |
2/51 |
3.9 |
|
Aortic dissection |
0/51 |
0.0 |
|
Incidental findings |
|
|
|
Mitral regurgitation |
7/51 |
13.7 |
|
Tricuspid regurgitation |
2/51 |
3.9 |
|
BAV, bicuspid aortic valve; FDR, first-degree relative. #Abnormal aortic valve morphology includes BAV, quadricuspid aortic valve, thickened RCC and NCC. *BAV, mild aortic stenosis. 'TAV, moderate aortic regurgitation. |
||
Table 4: Incidence of BAV & its associated complications in FDR screened.
|
Parameters |
Bicuspid Aortic Valve (N=4), mean (SD) |
Tricuspid Aortic Valve (N=47) mean (SD) |
P value* |
|
LV EF (%) |
63.3 (5.92) |
64.3 (5.73) |
0.732 |
|
AV Max Velocity (m/sec) |
1.66 (0.97) |
1.26 (0.30) |
0.050 |
|
AV mean PG (mmHg) |
5.00 (3.34) |
3.67 (1.35) |
0.107 |
|
AVA (cm2) |
2.23 (0.84) |
2.41 (0.62) |
0.571 |
|
Aortic Root Diameter (mm) |
2.60 (6.10) |
26.7 (3.65) |
0.770 |
|
N, number of persons examined with TTE. *One-way ANOVA test for quantitative data. TTE, transthoracic echocardiography. LV EF, left ventricular ejection fraction. AV, aortic valve. AVA, aortic valve area. PG, pressure gradient. |
|||
Table 5: Relevant Transthoracic Echocardiographic (TTE) Parameters.
Citation: Kan CS, Zailani NH, Zuki R, Bujang MA, Soon SY, Hsun Jong Y (2022) An Interventional Echocardiographic Screening Program for First-Degree Relatives of Patients with Bicuspid Aortic Valve: Is It Practical. J Surg Insights: JSI-100036