Annals of Pediatrics and Child Care
The Case of Fetus Neural Tube Defect: Spina Bifida with Severe Hydrocephalus
Mindaugas Sakys1,2*, Dainius Wojczulis1,2, Zygimantas Macys2, Audrone Arlauskiene1,2, Diana Ramasauskaite1,2, Dalia Lauzikiene1,2, Jolita Zakareviciene1,2
1Vilnius University Hospital Santaros klinikos Centre of Obstetrics and Gynaecology, Lithuania
2Vilnius University Faculty of Medicine, Institute of clinical medicine, Clinic of Obstetrics and Gynaecology, Lithuania
*Corresponding author: Mindaugas Sakys, Vilnius University Hospital Santaros klinikos Centre of Obstetrics and Gynaecology, Universiteto g. 3, Vilnius 01513, Lithuania, Tel: +37064454944.
Citation:Sakys M, Wojczulis D, Macys Z, Arlauskiene A, Ramasauskaite D, et al. (2019) The Case of Fetus Neural Tube Defect: Spina Bifida with Severe Hydrocephalus. Ann Pediatr Child Care: APCC-100002.
Received Date: 20 January, 2019; Accepted Date: 11 February, 2019; Published Date: 26 February, 2019
1. Abstract
Neural tube defects are congenital spinal cord, spine or brain malformations. Clinical manifestation of neural tube defects varies from mild form to severe - anencephaly, which is fatal. We present a case of a newborn with spina bifida and severe hydrocephalus.
2. Keywords:Hydrocephalus; Myelomeningocele;Neural tube malformation;Spina bifida
3. Introduction
Neural tube defect (NTD) is a result of dysfunction in neurulation process between third and fourth week of gestation. Myelomeningocele (MMC) is the most common NTD. It is characterized by a cleft in the vertebral column, with a corresponding defect in the skin so that the meninges and spinal cord are exposed. This can lead to a sensory function loss, lower limb paralysis, bladder and bowel dysfunction. Incidence of spina bifida is 1 of 1000 newborns [1]. The etiology is multifactorial, starting with genetic mutations to an inadequate nutrition. Spina bifida is surgically treatable malformation usually by closuring the spinal cord with spinal deformity reconstruction. This defect is compatible with long-term survival, however upper vertebral column spina bifida is associated with severe disability even after successful operation.
Hydrocephalus is a common disorder of cerebral spinal fluid (CSF) physiology resulting in abnormal expansion of the cerebral ventricles and increased intraventricular pressure. Infants commonly present with progressive macrocephaly whereas children older than 2 years generally present with signs and symptoms of intracranial hypertension. The classic understanding of hydrocephalus as the result of obstruction to bulk flow of CSF is evolving to models that incorporate dysfunctional cerebral pulsations, brain compliance, and newly characterized water-transport mechanisms. Congenital hydrocephalus, most commonly involving aqueduct stenosis, has been linked to genes that regulate brain growth and development [2]. The incidence of a congenital hydrocephalus is 2-3 of 5000 pregnancies [3].
Deficiency of a folic acid leads to higher risk of NTD. Studies shown that folic acid supplementation before getting pregnant reduces incidence of NTD by 71-90% and during the pregnancy period by 70% [4]. Folic acid supplementation is highly recommended to be 0.4 mg/day until 12th week of pregnancy for low risk women and for high risk pregnancies (diabetes mellitus, obesity, family history with NTD’s, epilepsy etc.) to be 1-5 mg/day [5].
4. Case Report
A 28-year-old pregnant woman (gravida 2, para 2) was referred to perinatology center for examination at 33 weeks and 6 day of gestation.
Ultrasonography (USG) examination revealed fetal hydrocephalus, spina bifida with MMC 3,1cm at lumbo-sacral region, polyhydramnios (Figure 1). Fetus biometry - BPD: 11 cm; FO: 15.0 cm; HC: 42.6 cm; AC: 29.0 cm; FL: 6.4 cm; Hum: 5.2 cm. Estimated weight: 2831 g ± 413; AFI: 25.1; Both cerebral hemisphere parenchyma thickness 13 mm. Placental attachment - dorsally. HR 122 b/min; umbilical artery S/D ratio: 2.4; RI: 0.59.
Obstetric and family history was uneventful. Diagnostic amniocentesis revealed a 46, XX karyotype.
Magnetic resonance imaging (MRI) confirmed the pathological findings made by USG (Figure 2):
• Intracranial cavity filled with liquid, from all cerebral structures only cerebral hemispheres/F lobes parenchymal layer ventrally about 13 mm thick is seen, which caudal part goes to mesencephalon and brainstem (IV ventricular up to 7 mm width). Falxcerebri could be identified. Cranial AP diameter sagittal up to 161 mm, transversally ~120 mm.
• Chiari 2 malformation should be distinguished, because lower brainstem and cerebellar tissue extend caudally through the foramen magnum into the cervical spinal canal.
• Signs of spina bifida are seen in the distal lumbal part (~23 mm length) around ~12 mm width meningocelle signs.
• Lumbo-sacral cyphosilic angle ~70°.
Due to a big head circumference and breech presentation, neonate was delivered at the 38th week of gestation by the caesarean section. Female newborn weight- 4300 g, 50 cm height. Head circumference was 53 cm. Apgar scores is 7/8 out of 10, umbilical artery pH - 7.32.
After the birth neurosurgeon’s consultation was provided: decompensated hydrocephalus, brain parenchymal thickness approximately 13 mm. Myelomeningocele in lumbosacral part was covered with thin layer membrane, 6 cm x 4.5 cm size without liquorea. Neurological evaluation revealed a full spinal cord violation with the lower-extremities paraplegia and a pelvic organs dysfunction.
Heart and kidneys were without pathological changes. The external ventricular drain was inserted on the second day of life. After two weeks the external ventricular drain was replaced with ventriculoperitoneal shunt. The patient was sent home one month after replacement of ventriculoperitoneal shunt with the monthly neurosurgeons and neonatologist follow up.
5. Discussion
In infants, hydrocephalus without an obvious extrinsic cause is usually referred to as congenital hydrocephalus, since it is often present at birth. When hydrocephalus occurs as a complication of another condition such as hemorrhage, infection or neoplasm, it is usually called acquired or secondary hydrocephalus. However, forces such as hemorrhage and infection can act prenatally and also cause “congenital” hydrocephalus [6].
There are a couple of most common causes speaking about congenital hydrocephalus. One of them is neural tube defects.The majority of patients with myelomeningocele have hydrocephalus. In this setting, hydrocephalus is caused by the Chiari II malformation which obstructs outflow of CSF from the fourth ventricle and/or flow through the posterior fossa. In addition, there is often associated aqueductal stenosis. Hydrocephalus associated with myelomeningocele tends to have both an obstructive component and a communicating component [7].
Isolated hydrocephalus is frequently caused by aqueductal stenosis. This can be due to congenital narrowing of the aqueduct or can result from inflammation due to intrauterine infection [8].
Central nervous system malformations are frequently associated with hydrocephalus. One of them Chiari malformation, which often accompanies a neural tube defect, portions of the brainstem and cerebellum are displaced caudally into the cervical spinal canal and lower brainstem is longer than normal, kinked and microscopically dysplastic. This obstructs the flow of CSF in the posterior fossa, leading to hydrocephalus. The Chiari II malformation seen in spina bifida is acquired and is accompanied by other features on MRI, such as agenesis of corpus callosum, low lying torcularherophili, tectal breaking, medullary kinking, and heterotopias [8].
The Dandy-Walker malformation consists of a large posterior fossa cyst that is continuous with the fourth ventricle and defective development of the cerebellum, including partial or complete absence of the vermis. Hydrocephalus develops in 70 to 90 percent of patients with Dandy-Walker malformation [8].
Hydrocephalus also could be, because of a structural anomaly of the brain - holoprosencephaly. Which often includes aqueductal stenosis or atresia and causes hydrocephalus, that can occur and may result in macrocephaly [9].
A vein of Galen malformation is a rare cause of hydrocephalus. The hydrocephalus in these patients is primarily caused by arterial pressure in the venous system rather than by compression of the aqueduct [8].
There are several genetic disorders that cause hydrocephalus. For example - L1CAM gene mutation, which is mapped to Xq28 [6]. X-linked hydrocephalus associated with stenosis of the aqueduct of Sylvius (HSAS) is the most common heritable form of hydrocephalus, thought to account for up to 10% of males with isolated idiopathic hydrocephalus. Mutations in L1CAM are the main genetic cause of HSAS, which occurs within a broader spectrum of disease that also includes isolated agenesis of the corpus callosum as well as X-linked spastic paraplegia. The L1CAM gene product is a neural recognition molecule that plays key roles in neuronal migration and axon guidance. When mutated, it gives rises to several structural malformations that obstruct CSF flow, most commonly at the level of the aqueduct [6].
Other common genetic cause is Fried syndrome, was once thought to be an example of L1 syndrome before it was recognized as a separate X-linked disorder [6]. Now known to be caused by mutations in the AP1S2 gene, Fried syndrome is characterized primarily by intellectual disability with prominent basal ganglia iron deposition or calcifications on CT scan (which may not be obvious on MRI), and variable hydrocephalus. Mutations in the same gene have now been recognized as the cause of other overlapping X-linked intellectual disability syndromes that are often accompanied by hydrocephalus. The severity and radiographic appearance of the hydrocephalus associated with mutations in AP1S2 has not been well characterized, but has been described as aqueductal stenosis [6].
Another syndromic forms associated with hydrocephalus can include trisomies 13, 18, 9, and 9p, as well as triploidy[10]. Rare autosomal recessive disorders include type 2 lissencephaly syndrome, which is also characterized by ocular anomalies, and hydrolethalus syndrome, which is associated with micrognathia, postaxial polydactyly of the hands, and preaxial polydactyly of the feet [8].
Therefor intrauterine infections such as rubella, cytomegalovirus, toxoplasmosis, lymphocytic choriomeningitis, syphilis, and Zika virus can result in congenital hydrocephalus. The mechanism is inflammation of the ependymal lining of the ventricular system and the meninges in the subarachnoid space [10]. This may lead to impaired absorption of CSF and/or to obstruction of CSF flow through the aqueduct or basal cisterns [7].
Nowadays there are lots of ways to treat MMC. The Management of Myelomeningocele Study (MOMS) trial showed a significant reduction of ventriculoperitoneal shunt placement at one year of age following fetal surgery (prenatal group: 40%; postnatal group: 82%) [1]. MMC is associated with severe disabilities throughout life. Therefore, nowadays MMC repair operations in-utero are provided and results are very promising. The aim of the in-utero operation is to avoid secondary neural tissue damage by amniotic fluid. Infants in the prenatal-surgery group were more likely to have a level of function that was two or more levels better than expected according to the anatomical level (32% vs. 12%) and less likely to have a level of function that was two or more levels worse than the expected level (13% vs. 28%) than were infants in the postnatal-surgery group. Although the ability to walk is dependent on lesion level, children in the prenatal-surgery group were more likely than those in the postnatal-surgery group to be able to walk without orthotics or devices (42% vs. 21%). The prenatal-surgery group had better motor function than the postnatal-surgery group, even though those in the prenatal-surgery group had more severe anatomical levels of lesions. But there were no significant differences between-groups in cognitive scores [11]. Hindbrain herniation was also significantly reversed in the fetal surgery group.
Nevertheless, fetal surgery was associated with significant risks related to premature birth. Moreover, about 25% of mothers in the fetal surgery group demonstrated evidence of thinning of the uterine wound at the time 10% of cesarean delivery, and showed partial (9%) or complete (1%) tissue edge separation at the hysterectomy site, although none had a hysterectomy rupture [1]. Pregnancy complications were more common among women in the prenatal-surgery group. Maternal morbidity and pregnancy complications that were related to prenatal surgery included oligohydramnios, chorioamniotic separation, placental abruption, and spontaneous membrane rupture [11].
One of the procedures of liquor drainage to avoid hydrocephalus is choroid plexus coagulation. It effectively treats severe hydrocephalus and hydranencephaly in approximately 40% of cases and should be considered as an alternative to placement of a VP shunt for infants with these conditions [12].
Another procedure that reduces CSF is ventriculoperitoneal shunt operation. A retrospective cohort study, based on established protocol for VP shunt implant in hydrocephalic children with MMC showed that parameters used to guide the indication of VP shunts included measurement of head circumference (HC), evaluation of fontanels, and measurement of lateral ventricular atrium (LVA) width by transcranial USG. In conclusion, VP shunt is required in children with increased HC (≥ 2 standard deviation regarding age group), bulging fontanels, or LVA width of ≥ 15 mm after the closure of MMC [13]. Nevertheless, ventriculoperitoneal shunting have many complications. Abdominal complications include intestinal obstruction, volvulus, peritonitis, peritoneal cyst, CSF ascites, as well as migration of the distal catheter via the intestinal tract, umbilicus, scrotum, and vagina [14].
Despite advances in prenatal operative repair, MMC still produces devastating neurologic deficits. The amniotic membranes (AM) are a biologically active tissue that has been used for human fetal MMC repair. This study evaluated the use of autologous AM compared to skin closure in an established fetal MMC model. These results suggest a potential role for AM in fetal MMC repair that warrants further study [15].
Diagnosis of hydrocephalus, can be determined in utero by USG as early as 12 weeks of gestation or later with MRI. Postnatally USG is helpful, brain MRI remains the best diagnostic test [16].
One of the laboratory methods to suspect NTD is maternal serum alpha-fetoprotein (AFP) testing in a triple screen or quadruple screen test. Elevated levels ofAFP suggests the open neural tube defects like spina bifida. That's typically a result of 2.5 times the average of AFP you'd expect to see at that point in your pregnancy. Therefore, triple screen or quadruple screen test and especially AFP testing and USG are very important in the prenatal diagnostics of NTD’s, genetic disorders or abdominal wall defects [17].
6. Conclusion
We have reported a unique case of a newborn with severe hydrocephalus and myelomeningocele. Multidisciplinary medical team (obstetricians, perinatologists, neonatologists, neurologist and neurosurgeon) were working in close relations.
In this case report we would like to highlight these medical care steps:
1. Earlier as much as possible diagnostics of neural tube defects in order to provide the best child delivery strategy.
2. Multidisciplinary post-delivery care of newborn (follow-up of vital functions and adequate neurosurgical care should be provided).
3. Further care of living child is essential.

Figure 1: USG images at 33 weeks + 6 days of gestation showing the fetal hydrocephalus. Biparietal diameter – 11.4 cm, occipitofrontal diameter - 15.0 cm, head circumference – 42.6 cm. (a). Red arrows indicate spina bifida defect at lumbo-sacral region (b and c).
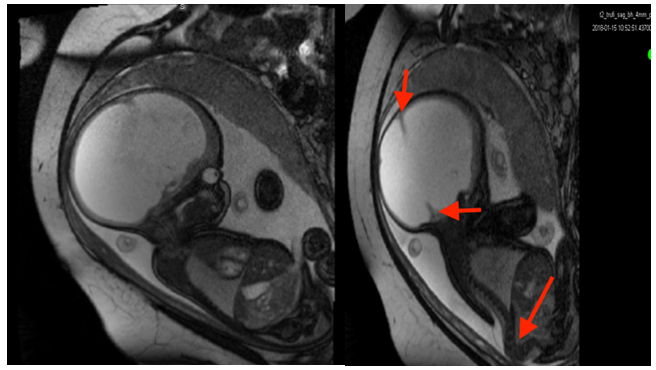
Figure 2: Fetal T2 weighted MRI sequence in sagittal plane at 36 week + 6 days of gestation. Intracranial cavity filled with liquid. (a) Small red arrows indicate falxcerebri and longer arrow indicates abnormallumbo-sacral cyphosilic angle. (b)Follow-up USG scans every 2 weeks were performed.
Citation:Sakys M, Wojczulis D, Macys Z, Arlauskiene A, Ramasauskaite D, et al. (2019) The Case of Fetus Neural Tube Defect: Spina Bifida with Severe Hydrocephalus. Ann Pediatr Child Care: APCC-100002.