Current Trends in Biotechnology and Biochemistry
Research Article
Arachis hypogaea histone deacetylase1 (Ahhda1) is involved in the Epigenetic Regulation of the Drought Stress Response
Bo Hu, Ling Li*
Guangdong Provincial Key Laboratory of Biotechnology for Plant Development, School of Life Sciences,South China Normal University, China
*Corresponding author: Ling Li, Guangdong Provincial Key Laboratory of Biotechnology for Plant Development, School of Life Sciences, South China Normal University, 510631, China. Tel: +862085211065.
Citation: Li L and Hu B (2019) Arachis hypogaea histone deacetylase1 (Ahhda1) is involved in the Epigenetic Regulation of the Drought Stress Response. Curr Trends Biotechnol Biochem: CTBB-100001
Received Date: 23 December, 2018; Accepted Date: 07 January, 2019; Published Date: 13 February, 2019
1. Abstract
Epigenetic control of chromatin structure plays a critical role in plant stress responses. Thus, histone density and various histone modifications are involved in the response to both drought and the phyto hormone abscisic acid (ABA) in various plants. Particularly important in this context is histone acetylation, and in this review the recent progress in understanding the part played by histone deacetylase1 (AhHDA1) from peanut (Arachis hypogaea) in the epigenetic regulation of the drought stress response was summarized. We also explore the prospects for future research in this field. In addition, we explore how plants are able to manipulate epigenetic regulation to enhance their stress tolerance, as well as how histone modifications in drought stress involved in the regulation of gene expression.
2. Keywords: AhHDA1; epigenetic regulation; peanut; drought stress
3. Introduction
Plants are often subjected to abiotic stress, such as drought stress, which limits cell growth and development, and consequently reduces plant growth and crop productivity. Peanut (Arachis hypogaea), which is an important cultivated source of edible oil, is typical in this regard, since local drought stress can severely limit its yield. The diverse strategies developed by plants to cope with drought stress [1] centre around abscisic acid (ABA), a key plant stress-signaling hormone that modulates physiological responses [2].
Many processes are involved in the biosynthesis of ABA, but the oxidative cleavage of cis-epoxy carotenoids by 9-cis-epoxy carotenoid dioxygenase (NCED) is considered to be the rate-limiting step [3]. During drought stress, drought-resistance genes are regulated by components of the ABA signaling pathway in plants and this regulation involves a number of transcription factors, including ABA-responsive element (ABRE) binding proteins [4]. In peanut, we found that AhAREB1, an ABRE-binding protein [5], was the key factor in AhNCED1 feedback regulation. AhNCED1 expression is partly inhibited by a complex of AhAREB1 and another transcription factor, AhNAC2. ABA enhances both the synthesis and degradation rate of AhNAC2, but slows AhAREB1 degradation, resulting in its accumulation. Accordingly, the AhAREB1/AhNAC2 protein complex functions as a negative feedback regulator of drought-induced ABA biosynthesis in peanut [5].
The post translational modification of histones is a common means of epigenetic modification of chromatin that can involve acetylation and ubiquitination of lysine residues, methylation of arginine, and phosphorylation of serine or threonine [6,7]. This regulates the expression of genes within the modified chromatin, and can, for example, affect plant growth and development [8,9]. Histone acetylation is controlled by histone acetyl transferases (HATs) and histone deacetylases (HDAs or HDACs). In peanut, we have characterised A. hypogaea histone deacetylase1 (AhHDA1) and have demonstrated its involvement in the epigenetic regulation of the drought stress response [10]. While there are very few reports in any crop species on the involvement of chromatin deacetylation in the response to osmotic stress, and in particular ABA signal transduction, we and others have begun to explore these relationships in peanut and we present a review of some of the recent findings below.
4. Water stress and ABA affect the acetylation of histone H3 in peanut seedlings
Histone acetylation is an important epigenetic modification, which regulates gene activity in response to stress. Based on sequence similarity, substrate specificity, and cofactor requirement, plant HDACs are classified into three distinct families, namely RPD3/HDA1-likeHDAs, SIR2-like HDAs, and HD2 proteins [11-14]. These HDAs are involved in plant development or the response to environmental stress. Although there has been considerable progress in understanding histone acetylation in other plants, there was no information in peanut prior to our work. We first isolated and characterized AhHDA1, anRPD3/HDA1-like super family histone deacetylase (HDA) gene from A. hypogaeaL, and found this gene to be up regulated by PEG-induced water limitation and ABA signaling. AhHDA1 possesses HDAC activity and is very similar to the Arabidopsis HDAC, AtHDA6 [10]. .AhHDA1 transcript levels are enhanced following treatment with PEG, ABA, and the specific HDAC inhibitor trichostatin A (TSA), indicating that AhHDA1 might be involved in the epigenetic regulation of stress resistance genes that comprise the responses to water stress and ABA [10]. A detailed examination of the effect of drought and ABA on chromatin showed an increase in histone H3 acetylation on the lysine residues at positions 9 and 14, respectively. The drought-resistance-related genes AhAREB1, AhDHN2 and AhNCED1 were also induced to various degrees by PEG and ABA treatment, while AhAREB1, AhDREB1, AhDHN2 and AhNCED1 chromatin was significantly enriched in H3K14ac by 100 µΜ ABA treatment [10]. These results indicate that drought and ABA might augment the expression of drought-resistance-related genes by increasing histone acetylation in key chromatin domains. Regions of the AhNCED1 promoter are increasingly enriched with AhHDA1 protein when peanut leaves were treated with 20% PEG or 100 µMABA [15]. Furthermore, AhHDA1 limits induction of their porter construct pAhNCED1::LUC by ABA. The results indicate that AhHDA1 is involved in ABA synthesis feedback inhibition by limiting AhNCED1 expression in response to increased ABA levels. The fluorescence intensity of plants transformed with pAhAREB1: :LUC is significantly reduced when co-transformed with 35S::AhHDA1, and this reduction is enhanced by treatment with 10 µMABA [10]. These results indicate that AhHDA1 suppresses the transcription of the AhAREB1 and AhSnRK1-like genes, and that exogenous ABA application facilitates this effect of AhHDA1.
AhHDA1 participates in ABA signal transduction by negatively regulating the promoter activity of AhAREB1 and AhSnRK1-like. We also found that AhHDA1 may change the cell shape and development level by influencing the related genes on biosynthetic pathways, which lead to the weaker drought resistance of over-expressed hairy roots; AhHDA1 can interact with AhAREB1 and negatively regulate the AhAREB1 and AhSnRK1- like genes, which are critical factors in the ABA signal pathway, and which participate in AhNCED1 transcription feedback during drought stress in peanut. This research highlights the biological significance of AhHDA1 in the response to drought and ABA signals, and lays the foundation for further detailed investigations into AhHDA1 function.
5. AhDREB1 expression, which is affected by histone acetylation, increases abscisic acid sensitivity and tolerance to water stress
The growth and development of plants can be optimized by regulating the expression of numerous stress responses via two main pathways, ABA-dependent and ABA-independent [16]. However, some researchers suggest that the two types of signaling pathway may be interdependent due to cross-talk between them [17]. In the ABA-independent pathway, dehydration-responsive element binding protein (DREB) regulates many drought-responsive genes, such as RD29A, P5CS1 and SODs, by binding to dehydration responsive element (DRE) motifs, which comprise nine conserved bases (TACCGACAT), in their promoter regions. This activates the respective genes and thereby increases the plant’s drought resistance [18,19].
Acetyl groups are removed from acetylated histones by HDACs, whose activity is generally related to transcriptional repression and gene silencing [20]. As a molecular inhibitor of HDAC, TSA cause transient increases in the acetylation of histones H2B, H4 and H3 in chromatin [21]. We isolated a full-length coding sequence of the AP2/ERF family gene AhDREB1 from peanut and found that exogenous ABA and PEG induce AhDREB1 expression [22]. TSA can significantly up regulate the expression of AhDREB1, implying that histone acetylation might be involved in its transcriptional regulation. Consistent with these results, we found that, following TSA treatment, the levels of H3ac were significantly increased in the P4 and P5 regions of AhDREB1 chromatin [22]. Clearly, therefore histone acetylation is involved in the transcriptional regulation of AhDREB1.
Many studies have demonstrated that histone acetylation is important for the adaptation of plants to abiotic stress. For example, in Arabidopsis, the RD29A, RD29B, RD20 and RAP2.4 genes are induced by drought, and this correlates with elevated levels of H3K4me3 and H3K9acin the histones associated with the coding regions of these genes [23]. In maize, expression of the cold-induced ZmDREB1gene is increased by HDAC activity, and this then affects plant tolerance to cold stress [24]. During PEG and ABA treatment, the acetylation of H3K9 and K3K14 is increased in A.hypogaea [10], but by modulating the histone acetylation levels of stress-responsive genes, AtHDA9 negatively affects plant sensitivity to drought and salt stresses in A. thaliana [25]. In Arabidopsis plants over expressing AhDREB1, both ABA levels and ABA sensitivity are increased, both of which affect the ABA signaling pathway and ultimately cause an increase in expression of the downstream drought stress-related genesRD29A, P5CS1, P5CS2 and NCED1 [22]. Thus, in Arabidopsis, AhDREB1can enhance tolerance to drought via the ABA-dependent pathway. The involvement of histone acetylation in this effect is demonstrated by TSA treatment, which promotes AhDREB1 transcription concomitant with enrichment of H3acin regions of the AhDREB1 gene [22]. In conclusion, the onset of water stress leads to an increase in H3ac levels, which regulate AhDREB1 transcription, resulting in improved drought resistance of the plant. This is the first report that histone acetylation is involved in the transcriptional regulation of a DREB subfamily gene in peanut.
6. AhGLK, which interacts with AhHDA1, participates in the drought response and leaf growth after rehydration
Using a yeast two-?hybrid approach, AhHDA1 was shown to interact with AhGLK, a peanut homologue of Golden2-?like transcription factors found in many land plants. Thus, AhGLK contains a MYB domain that shows 60% identity to that of GsGLK, a counterpart in Glycine soja.' Interaction between AhHDA1 and AhGLK was confirmed by GST pull-down and bimolecular fluorescence complementation experiments [26]. AhGLK is located in the nucleus and, like other GLK family members, is a transcription factor associated with chlorophyll synthesis. Tissue analysis showed that AhGLK accumulates mainly in leaves, and its expression is down regulated by treatment with 30% PEG. Therefore, AhGLK participates in the drought response and it is also involved in the process of leaf growth after rehydration.
AhGLK1 affects chlorophyll biosynthesis and photosynthesis in peanut leaves during recovery from drought by regulating AhPORA (which encodes a key enzyme in chlorophyll biosynthesis) and AhCAB (which encodes a chlorophyll A/B binding protein). AhHDA1 has an antagonistic effect on this process, possibly by interacting with AhGLK1under drought conditions to reduce the expression of AhPORA and AhCAB. During recovery from water limitation, AhHDA1 levels decrease while AhGLK1 levels increase. AhGLK1 then enhances the expression of AhPORA and AhCAB by binding to their promoters.
7.Conclusions
AhHDA1 suppresses the transcription of the AhAREB1 and AhSnRK1-like genes, and that exogenous ABA application facilitates this effect of AhHDA1. The involvement of histone acetylation in this effect is demonstrated by TSA treatment, which promotes AhDREB1 transcription concomitant with enrichment of H3acin regions of the AhDREB1gene. AhGLK1 is regulated by itself and activates AhCAB and AhPORA expression via binding to their promoters, while AhHDA1 may attenuate the transcriptional activity of AhGLK1 by interacting with AhGLK1 (Figure 1).
8. Perspective
Recent research shows that AhHDA1, which maintains a high level of expression and activity during drought stress and ABA treatment, is likely involved in feedback regulation of the ABA signaling pathway. High AhHDA1 expression levels may inhibit cascade amplification of the ABA signaling pathway, and then maintain its dynamic balance. Adjusting the key components of ABA signaling regulates the sensitivity of plants to ABA and one means of achieving this is by histone acetylation / deacetylation modification of relevant genes. The consequent regulation of downstream gene expression can modulate the stress tolerance of plants. Furthermore, AhHDA1 participates in the regulation of drought stress memory [27]. Future studies will focus on identifying the most important genes in both the ABA-dependent and ABA-independent signaling pathways that undergo specific histone acetylation in response to water limitation and ABA treatment. Such studies should help elucidate the molecular mechanisms of epigenetic regulation of the drought stress response by histone acetylation in peanut.
9. Author Contributions
Both BH and LL wrote the article.
10. Funding
The work described in this review was funded by the National Natural Science Foundation of China (grantno.31471422, awarded to LL), the Natural Science Foundation of Guangdong Province (grantno.2018A131303629, awarded to BH), and the Science and Technology Project of Guangzhou (grant n.o 201804010158, awarded to BH).
11. Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
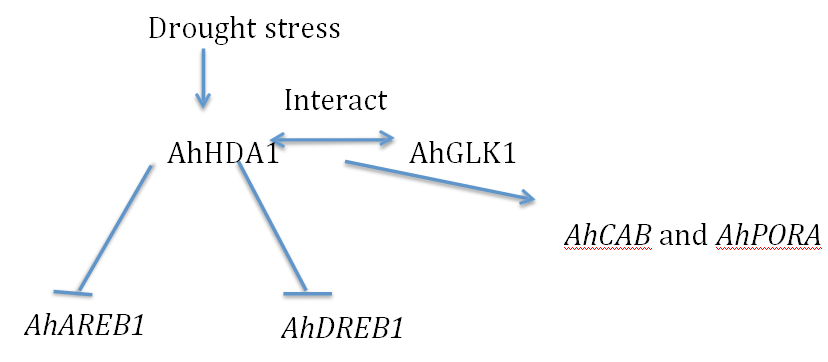
Figure 1: AhHDA1 is involved in the epigenetic regulation of the drought stress response
euchromatic regions and demonstrates histone deacetylase activity in vitro. Cell Res. 16: 479-488.
Citation: Li L and Hu B (2019) Arachis hypogaea histone deacetylase1 (Ahhda1) is involved in the Epigenetic Regulation of the Drought Stress Response. Curr Trends Biotechnol Biochem: CTBB-100001