Journal of Surgery and Insights
ISSN 2652-4643
Case Study
Pilot Study to Assess Pre-Operative use of Botulinum Toxin to Improve Post-Operative Compliance with Rehabilitation in Patients with Upper Limb Spasticity Undergoing Tendon Lengthening/Transfer: A Case Series
Gill P1*, Henry A1, Hassan Z1,2 and McArthur P1,2
1Department of Burns and Plastic Surgery, Whiston Hospital, Mersey, United Kingdom
2Department of Burns and Plastic Surgery, Alder Hey Children’s Hospital, Liverpool, United Kingdom
*Corresponding author: Gill P, Department of Burns and Plastic Surgery, Whiston Hospital, Warrington Road, Prescot, L35 5DR, United Kingdom.
Citation: Gill P, Henry A, Hassan Z, McArthur P (2020) Pilot Study to Assess Pre-Operative use of Botulinum Toxin to Improve Post-Operative Compliance with Rehabilitation in Patients with Upper Limb Spasticity Undergoing Tendon Lengthening/Transfer: A Case Series. J Surg Insights: JSI-100030
Received date: 15 October, 2020; Accepted date: 20 October, 2020; Published date: 27 October, 2020
Abstract
Introduction: Botulinum toxin (BTx-A) is used in upper limb spasticity to interrupt nerve stimulation by inhibiting the presynaptic release of acetylcholine at the neuromuscular junction. This case series presents the use of BTx-A pre-operatively before definitive tendon lengthening or transfer, in order to optimize the post-operative rehabilitation regime.
Methods: Patients due to undergo tendon lengthening or transfer were identified by the lead surgeon and administered BTx-A to the affected muscles under ultrasound guidance 2-4 weeks pre-operatively. Following definitive surgical management, immediate aggressive hand therapy regime was commenced and patients were followed up in outpatient clinic at 3 months.
Results: 5 cases have been presented in this case series. All cases exhibited reduced painful spasticity upon waking up from the general anesthetic in the definitive tendon lengthening/transfer procedure, thus reduced tension across the coaptation and reduced analgesic requirement. This enabled greater compliance with the splint used post-operatively and enabled the hand therapist to proceed with an aggressive hand therapy regimen resulting in better outcomes.
Conclusion: Our case series provides evidence to support the use of BTx-A to pre-condition patients presenting with upper limb spasticity before definitive surgical management. The implication for clinical practice is reduction in immediate post-operative spasticity, leading to reduced pain experienced by the patient and a greater range of function, encouraging increased compliance with splintage.
Keywords: Botulinum toxin; Rehabilitation; Upper limb spasticity
Introduction
Spasticity can be defined as a velocity-dependent resistance, produced when stretching muscle [1]. Upper limb spasticity can result from upper motor neuron injuries such as traumatic brain injury (TBI), cerebrovascular accidents (CVA) or cerebral palsy (CP) [2]. It impairs upper limb function with the additional adverse effect in children as it can also decrease longitudinal muscle growth, thereby
leading to a permanent deformity [3].
The management of patients with upper limb spasticity should ideally be carried out within a multidisciplinary team consisting of surgeons, neurologists, hand therapists, occupational therapists and psychologists in order to address the complex issues surrounding the rehabilitation of the patient. Treatment consists of conservative, pharmacological and surgical measures depending on the severity of the upper limb spasticity and the potential for improvement in function. We have proposed an algorithm for the management of these patients and are currently awaiting its publication [4].
Botulinum toxin (BTx-A) injection in the upper limb has been shown to reduce spasticity and improve function in patients with TBI [5], CVA [6] and CP [7]. Patients may progress to development of a fixed contracture, resistant to any further improvement with BTx-A or hand therapy and unable to maintain a good resting position in a splint. The next step is to offer surgical intervention, in the form of tendon lengthening or tendon transfer [8]. We have noticed that following definitive surgical intervention, patients usually suffer with a refractory spasticity upon waking up from general anesthetic. This is observed to be very painful, commonly requiring increased levels of analgesia and often tolerance of splint application is poor.
Materials and Method
We propose using BTx-A under ultrasound guidance (Figure 1) to pre-condition the muscle before tendon lengthening or tendon transfer. The aim is to reversibly denervate the muscle 2-4 weeks pre-operatively, allowing dampening down of the refractory spasticity that occurs when the patient awakes from general anesthetic. This acts to reduce the postoperative pain experienced by the patient, enabling application and compliance with the splint [9]. The hand therapist can commence an early aggressive rehabilitation regime during the maximal effective period of the BTx-A to produce an optimal outcome for the patient.
Results
We present 5 cases in which we performed pre-conditioning using BTx-A prior to definitive surgical management with tendon lengthening and tendon transfer.
Case 1: A 17-year-old male with a background of CP presented with right sided upper limb spasticity affecting the right hand and right wrist. BTx-A was injected into his right pronator teres (PT), flexor carpi ulnaris (FCU), flexor carpi radialis (FCR), flexor digitorum profundus (FDP), flexor digitorum superficialis (FDS) and flexor pollicis longus (FPL) under ultrasound guidance. Two weeks later he underwent right FCU and FPL lengthening, with FCR transfer. A splint was applied with ease and he commenced early hand therapy. He maintained a good functional position with increased comfort on follow up in outpatient clinic at 3 months.
Case 2: A 16-year-old female with TBI presented with left sided upper limb spasticity affecting the left hand and left wrist (Figure 2). BTx-A was injected into her left PT, FCR, FDP, FDS, thenar muscles, interrossei muscles and adductor muscles. Two weeks later she underwent left FCR transfer to extensor carpi radialis brevis (ECRB) and tendon lengthening of FDP, FDS, FPL and FCU. A splint was applied and she commenced early hand therapy. At follow up in outpatient clinic at 3 months, it was noted that she maintained a good left wrist position at rest (Figure 3), however she required ongoing BTx–A injections to maintain position of her finger flexors.
Case 3: A 20-year-old female with TBI presented with right sided upper limb spasticity affecting her right hand and right wrist. BTx-A was injected into her right biceps, FCR and FDS. Two weeks later she underwent right FDS and FPL lengthening, with right FCR transfer to extensor carpi radialis longus (ECRL) and right 1st carpometacarpal joint fusion using kirschner wires. Application of a splint was performed and early hand therapy was initiated. Follow up in outpatient clinic at 3 months showed adequate range of movement from flexion (Figure 4a) to maximal extension, noting reduced extension of the right index finger at the distal interphalangeal joint (Figure 4b).
Case 4: An 18-year-old female with a background of CP presenting with left upper limb spasticity affecting the left hand and left wrist. Her main concern was reduced useful function for activities of daily living and cosmesis of the hand. BTx-A was injected into the left FCR, FDP, FDS and FPL. Two weeks later she underwent left FCR transfer to ECRB and tendon lengthening of FDS and FPL. A splint was applied and early hand therapy commenced. At 3 months follow up she was pleased with the increased range of useful hand function (Figure 5a,b) and also with the appearance of her hand.
Case 5: A 21-year-old male with a background of TBI presenting with left upper limb spasticity affecting the left elbow, wrist and hand. BTx-A was injected to his left biceps, brachialis, wrist extensors, FDS and lumbricals. Two weeks later he underwent lengthening of his left wrist extensors and release of the metacarpophalangeal volar plate and check rein ligaments. Follow up in outpatient clinic at 3 months revealed a good resting position with adequate extension of digits to enable hygiene management (Figure 6).
Conclusion
Our case series provides evidence to support the use of BTx-A to pre-condition patients presenting with upper limb spasticity 2 weeks prior to undergoing definitive surgical management. Patients display reduced post-operative refractory spasticity, resulting in pain relief and the ability to tolerate the application of the splint. In addition, there is reduced tension applied across the coaptation as the patient is more compliant when they wake up from the general anesthetic. The hand therapist is able to commence a more aggressive regime and push the patient to achieve greater ranges of movements in a more comfortable position.
Discussion
Patients with a background of CP, TBI or CVA may present to the hand surgeon with upper limb spasticity. These patients are at risk of developing painful flexion contractures, restricting function but also impairing the ability to carry out hygiene management leading to infections and maceration from nails digging into palms [10]. Traditionally neurologists, paediatricians, hand surgeons and therapists have carried out management of patients with upper limb spasticity. The management has been varied using conservative, pharmacological and surgical measures, with a recent streamlined algorithm (Figure 7) proposal awaiting publication [4] and the recommendation that patients should ideally be treated with a multidisciplinary team setting.
Botulinum toxin (BTx-A) is traditionally used to manage patients with upper limb spasticity, who have not yet developed a fixed flexion contracture. It is produced by Clostridium botulinum and it works by blocking the presynaptic release of acetylcholine at the neuromuscular junction, thus moderating the muscle contraction produced [11]. It is a method of reversible chemo denervation, which is dose-dependent and cumulative in effect [12]. It usually takes 5-7 days to take effect, peaks between 2 to 6 weeks and lasts up to 3 months, thus requiring repeat injections to maintain the desired effect [11].
Difficulties in maintaining hygiene, breakdown in palmar skin or restriction in pinch, grasp and release are all indications for surgical intervention [13]. Conservative measures are considered before surgical intervention, however there is no formal cut-off for degrees of deformity before surgery may be considered [8]. Athetosis is a relative contraindication to surgical intervention, as there are fluctuations in the degree of spasticity.
Surgical interventions to treat flexion contractures include step lengthening of tendons, tendon transfer, carpectomy and arthrodesis of wrist and selective neurectomy [14,15]. The lead author noticed increased postoperative spasticity and resultant pain in patients who underwent surgical intervention. It was more difficult to ensure these patients were compliant with splints due to the discomfort they experienced, leading to a delay in commencement of rehabilitation. The hand therapist noticed that patients were not reaching their full potential when undertaking hand therapy, thought to be due to the discomfort but also the increased spasticity, which
occurred as a result of the anxiety associated with previous pain. The idea that pre-conditioning using BTx-A could reduce the tension across the coaptation was the primary aim, however additionally the patients were noted anecdotally to have a marked reduction in pain immediately post-operatively. This has not been described in the current literature at present. We hope to undertake a study following this pilot, looking in more depth at post-operative pain scores using objective measures.
Patients with spasticity present in the pediatric and adult population, with evolving lifelong concerns with regards to upper limb function but also appearance. When devising a management plan for patients with upper limb spasticity, the goals of care must be discussed with the patient following assessment of the patient’s overall function. Realistic goals can be set in order to optimize functional outcome and meet patient expectations [16].

Figure 1: Injection technique under ultrasound guidance.
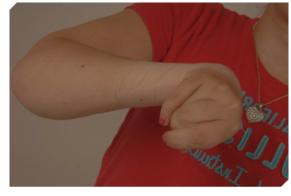
Figure 2: Case 2 pre-operative presentation.
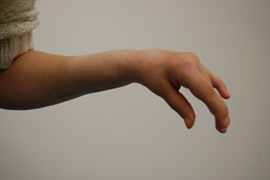
Figure 3: Case 2 follow up at 3 months.

Figure 4a: Case 3 follow up at 3 months with flexion.

Figure 4b: Case 3 follow up at 3 months with maximal extension, noting reduced extension of the right index finger at the distal interphalangeal joint.

Figure 5a: Case 4 grasp function.

Figure 5b: Case 4 lift function.
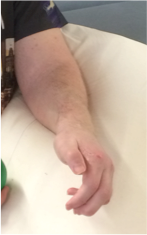
Figure 6: Case 5 post-operative position.
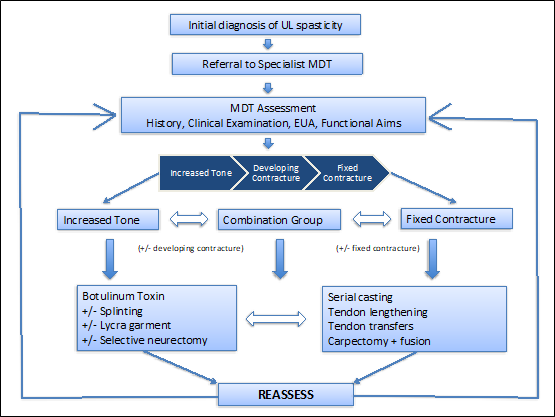
Figure 7: Algorithm for the management of patient with increased tone (Gill et al. 2020).
Citation: Gill P, Henry A, Hassan Z, McArthur P (2020) Pilot Study to Assess Pre-Operative use of Botulinum Toxin to Improve Post-Operative Compliance with Rehabilitation in Patients with Upper Limb Spasticity Undergoing Tendon Lengthening/Transfer: A Case Series. J Surg Insights: JSI-100030