Salvatore Alongi1, Raffaella Rosa1, 3, Ugo Murialdo1, Roberta Rissotto4 and Pia Allegri*1, 2
1Rapallo Hospital, Ophthalmic Department, Genova, Italy
2Rapallo Hospital, Ocular Inflammation Tertiary Referral Center, Genova, Italy
3University Eye Clinic, San Martino Hospital, Genova, Italy
4Catholic University of the Sacred Heart, Roma, Italy
*Corresponding author: Pia Allegri, Rapallo Hospital, Ophthalmic Department, Via San Pietro 8, 16033 Rapallo (Genova), Italy – Phone: +39 0185 683678 – Fax: +39 0185 683728.
Citation:Alongi S, Rosa R, Murialdo U, Allegri P (2019) Intravitreal Dexamethasone Implant (Ozurdex®) Injection Preceding Phacoemulsification in Patients Affected by Cataract and Diabetic Macular Edema: Our Preliminary Results. J DiabetesMetabManag: JDMM-100001.
Received Date: 04 January, 2019; Accepted Date: 17 January, 2019; Published Date:23 January, 2019
1. Abstract
1.1. Purpose: To evaluate the effectiveness of an intravitreal injection of slow-release dexamethasone implant (Ozurdex®) in patients affected by diabetic macular edema (DME) undergoing cataract surgery.
1.2. Methods: This was a prospective one-center study on 30 eyes of 23 diabetic subjects to assess the safety and efficacy of previous intravitreal administration of Ozurdex to prevent the worsening of a pre-existing DME after phacoemulsification. Primary outcomes were the variations on (1) - best-corrected visual acuity (BCVA) and (2) - central retinal thickness (CRT). Mean follow-up period was 6 months (± 9 days).
1.3. Results: Mean BCVA was 46.8 letters at baseline and it improved significantly 3 months after surgery (69.3 ETDRS letters – p< 0.001) and resulting stable (66.7 ETDRS letters) 6 months after cataract surgery. Mean CRT was 459μm at baseline and it decreased to 312 μm at six months from surgery (p< 0.001).Two cases of intra-ocular hypertension were the only recorded side-effect and both were successfully controlled by topical therapy.
1.4. Conclusions: Our study shows that intravitreal Ozurdex® injection one month approximately prior to cataract uncomplicated surgery, in patients affected by diabetic macular edema, is statistically effective and safe in order to prevent a worsening in DME and in visual acuity 6 months after phacoemulsification.
2. Keywords: Cataract; Dexamethasone intravitreal implant; Diabetic macular edema; Ozurdex®; Phacoemulsification;
3. Introduction
Diabetic patients develop cataract earlier than normal subjects and five times more frequently compared to the non-diabetic population. Multiple underlying pathogenetic mechanisms are responsible for lens alterations, among them: increase of osmotic phenomena related to polyol pathway activation, non-enzymatic glycation of lens proteins and increase in oxidative stress [1]. Macular edema is a severe complication of long-term uncontrolled diabetes mellitus leading to irreversible central vision damage. Moreover, it is well known that diabetic patients can present a worsening of a preexisting mild diabetic macular edema (DME) after cataract surgery; it usually increases 8-12 weeks following phacoemulsification and for such reason, this surgical procedure in diabetic patients is often delayed [2-3]. Until now no standardized treatment exists to improve or stabilize the visual outcome in DME patients after cataract surgery. Several strategies have been implemented in order to reduce DME incidence and worsening following phacoemulsification surgery, such as: better glycemic control, pre- and post-operative administration of topical NSAIDs, laser treatment [4], triamcinolone acetonide [5-7] or anti-Vascular Endothelial Growth Factor (anti-VEGF) intravitreal injection [8-9], but, they are usually ineffective in long-term because of a short antiedema effectiveness, an impairment in central vision or side-effects.
4. Materials and methods
This was a prospective study concerning patients affected by DME and advanced cataract treated at first with an intravitreal Ozurdex® injection followed, in a period of about 30 days (± 10 days), by standard uneventful phacoemulsification and simultaneous hydrophilic acrylic intraocular lens (IOL) in the capsular bag implantation using a 2.2 clear cornea tunnel and a viscoelastic device (Duovisc® Alcon).
Surgery was made by two operators working at the same Hospital (Rapallo Hospital – Genova). From April 2016 to November 2017, 30 eyes of 23 patients (14 females and 9 males whose mean age was 70 ± 3 years), met the inclusion criteria and were enrolled for the study.
Inclusion criteria were: non-proliferative diabetic retinopathy, clinically significant non-tractional DME, and visually significant cataract.
Exclusion criteria were: coexisting decompensated glaucoma despite therapy, previous intravitreal treatment with anti-VEGFs or corticosteroids in the 6 months before surgery, mature cataract which can obscure the fundus exploration, not well-controlled diabetes (glycated haemoglobin or HbA1C more than 10%) or blood pressure (≥ 140/85 mmHg), intraocular infectious or inflammatory diseases (uveitis) and intraoperative complications (such as vitreous loss or posterior capsular tear).
All patients underwent a comprehensive ophthalmic examination including measurement of best-corrected visual acuity (BCVA) by using a standardized ETDRS protocol, slit-lamp anterior and posterior segment examination, Goldmann tonometry, and dilated funduscopy at all follow-up visits.
Spectralis Optical Coherence Tomography (Heidelberg II Spectral Domain OCT) examination was carried out at baseline (T0) and at 3 and 6 months post-cataract surgery.
Intravitreal slow-release dexamethasone implant (Ozurdex®) injection was performed at T0 and was followed, 30 (±10) days, by phacoemulsification. Follow-up visits were scheduled at 1and 10 days, at 2, 3 and 6 months from T0 (Figure 1).
5. Results
We analyzed the data related to 30 eyes of 23 diabetic patients (14 females and 9 males) with coexisting cataract and clinical significant DME. Patient demographics are synthesized in (Table 1).
Mean BCVA at T0 (baseline) was 46.8 Early Treatment Diabetic Retinopathy Study (ETDRS) letters, at month 3 it was 69.3 ETDRS letters (p<0.01) and at month 6 it stabilized at 66.7 ETDRS letters (p<0.01) (Table 2).
Mean CRT was 459 ± 136.5μm at T0, 301 ± 97.3 μm at month 3 (p<0.01) and 312 μm ± 73 at month 6 (p<0.01) (Table 3).
Data analysis was performed by using T Student formula at 99th percentile. The only complication we registered was a transient intra-ocular hypertension (under 26 mmHg at Goldmann tonometer) in 2 eyes (6.7% of patients), which was controlled by topical antiglaucomatous treatment (carbonic anhydrase inhibitor + beta-blocker). IOP increase, was present at M2 visit (about 60 days from T0) and the 2 patients received pressure lowering medications, after which their IOP reached a stable level without the need for additional medical or surgical interventions.
(Figure 4a and 4b and Figure 5a and 5b) report some OCT pictures of two treated eyes which show the good results obtained at 6 months from Ozurdex® injection.
6. Discussion
Intravitreal dexamethasone implant (Ozurdex®; Allergan plc, Dublin, Ireland) is a slow-release biodegradable 0.7 mg-dexamethasone intravitreal implant, which is injected via pars plana with a 22-G specific injector. This drug shows several potential advantages in DME management. It has a wide range of action against different cytokines involved in the blood-retina barrier breakdown, which is an essential step in DME pathogenesis. Moreover, intravitreal continued release of small doses of anti-inflammatory steroidal drug lasting some months maximizes the therapeutic implant duration, reducing side-effects related to intraocular steroid administration and mainly providing a 3-4 month prolonged effect following phacoemulsification (useful to cover the 1-2 months period of proven DME worsening or relapse).
Recent literature reports proved the efficacy of dexamethasone slow-release implant in preventing the worsening of diabetic post-surgical macular edema.
The pivotal study MEAD (randomized sham-controlled 3-year follow-up clinical trial using Ozurdex® for DME treatment) showed that in diabetic patients who underwent cataract surgery there was an improvement in BCVA and no increasing in CRT after surgery [10].
A 3-year real-life study carried out on 128 eyes of DME patients treated with dexamethasone implant showed that patients who underwent cataract surgery (47% of the total number) did not present a BCVA reduction following phacoemulsification and these patients were treated with dexamethasone implant one month prior to the surgery [11].
This confirms the pivotal registration study MEAD data concerning the analysis of the patients’subset who underwent cataract surgery.
Few are the prospective studies on this topic and they show limited outcome numbers: Agarwal et al. [12] conducted a prospective study on 18 eyes affected by DME, 9 eyes received intravitreal dexamethasone implant at the beginning of phacoemulsification and reported a statistically significant result in BCVA improvement and CRT reduction compared to 9 eyes of the control group treated with phacoemulsification only after 24 weeks of follow-up. The Authors concluded that a single injection of Ozurdex® was able to control postoperative inflammation in a significative number of patients for 3–4 months and visual acuity improvement was related to a decrease in CRT.
In our study, we also observed a significant decrease in CRT at M3 visit (p<0.01) which was maintained at M6 visit although a minor increase in central retinal thickness, without a statistical importance, was detected; it may be the consequence of a therapeutical efficacy reduction with time.
A recent study by Panozzo et al. [13] proved the efficacy and safety of Ozurdex® implant injected at the end of phacoemulsification procedure in 19 eyes of 19 patients affected by naive or refractory DME confirming Sze [14] and Furino [15] data who reported encouraging results using dexamethasone implant at the end of cataract surgery.
Concerning simultaneous combined procedure, Agarwal chose to inject Ozurdex® implant before cataract surgery in order to avoid troubles in anterior chamber stability after cataract surgery, whereas Panozzo and Furino chose to perform it at the end of phacoemulsification to prevent disturbing glares during cataract surgery.
The purpose of our study was to investigate the safety and efficacy of Ozurdex® implant in DME suffering cataractous eyes undergoing phacoemulsification, performing the implant 30 ± 10 days before cataract surgery.
The rationale was to exploit the anti-inflammatory and anti-edema effect of dexamethasone implant to cover the common post-operatory macular inflammatory edema and to avoid the potential surgical risk of the injection into a hypotonic bulb at the end of phacoemulsification.
A recent report by Calvo et al. [16] showed very good short-term results on BCVA and CRT following the administration of Ozurdex® immediately after cataract surgery in 24 eyes of 24 diabetic patients showing that at 3 months follow-up mean CRT changed from 241.1 μm to 232.4 μm and mean BCVA improved from 20/50 at baseline to 20/25 at 3 months. Authors concluded that a single Ozurdex injection intraoperatively after phacoemulsification could avoid macular edema after cataract surgery in diabetic patients.
Our study is a 6-months follow-up study and confirms these short-term positive results in a more prolonged period. Ozurdex® treatment, in our experience, was free from persistent side-effects, which were only limited to ocular hypertension at visit M2 in 2 eyes of 2 different subjects; accordingly to Bahadorani et al. to explain it, these two eyes could be “latent” or unrecognized primary open angle glaucoma [17].
7. Conclusions
Slow-release intravitreal dexamethasone implant (Ozurdex®) approximately performed one month prior to phacoemulsification in patients affected by DME showed to be an effective and safe device in order to prevent macular edema worsening in patients affected by DME and undergoing cataract surgery, resulting in a statistically significant reduction in CRT, with a consequent improvement in BCVA which were maintained for six months following phacoemulsification. Additional comparative studies are required to determine the best therapeutical course for these patients and the most suitable timing to perform intravitreal dexamethasone implant injection.
8. Disclosure
The Authors report no conflict of interest and are responsible of the conduction of the paper.
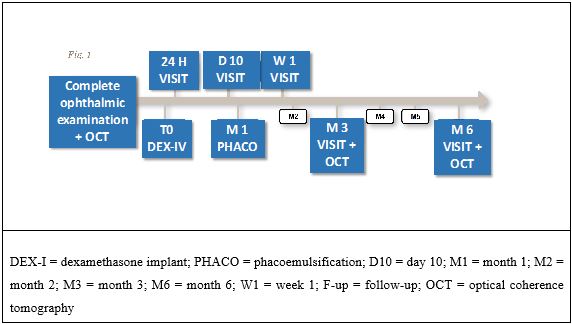
Figure 1: Timeline of visits and treatments
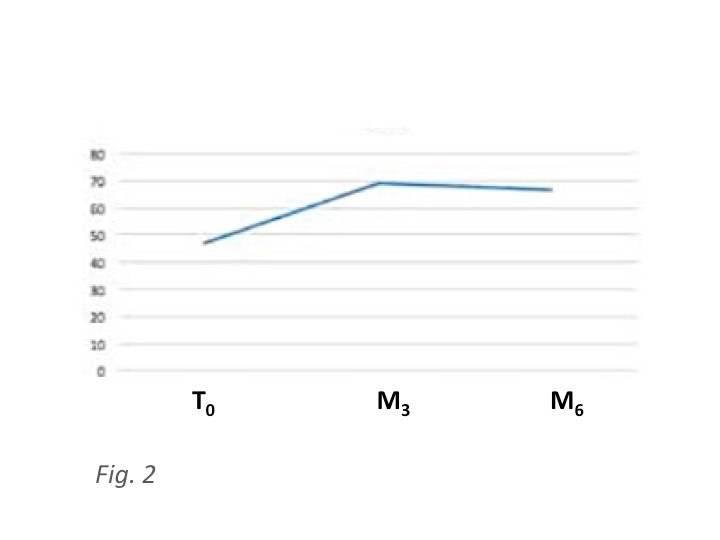
Figure 2: Best-Corrected Visual Acuity (BCVA) in ETDRS letters
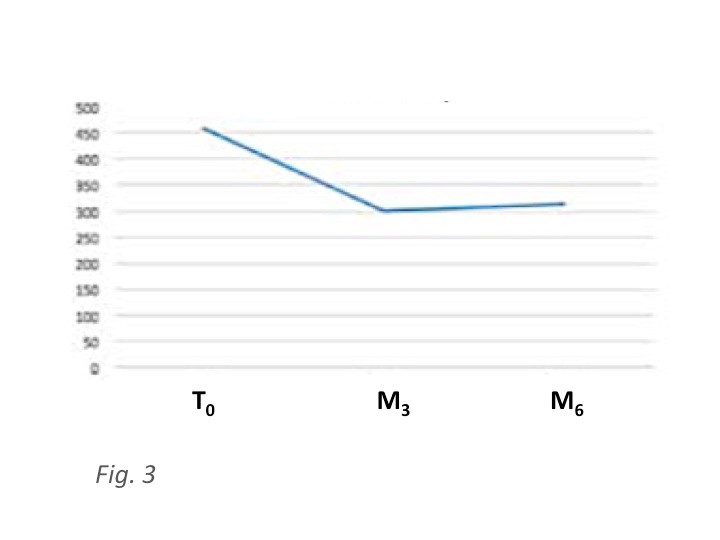
Figure 3: Central Retinal Thickness (CRT) in μm
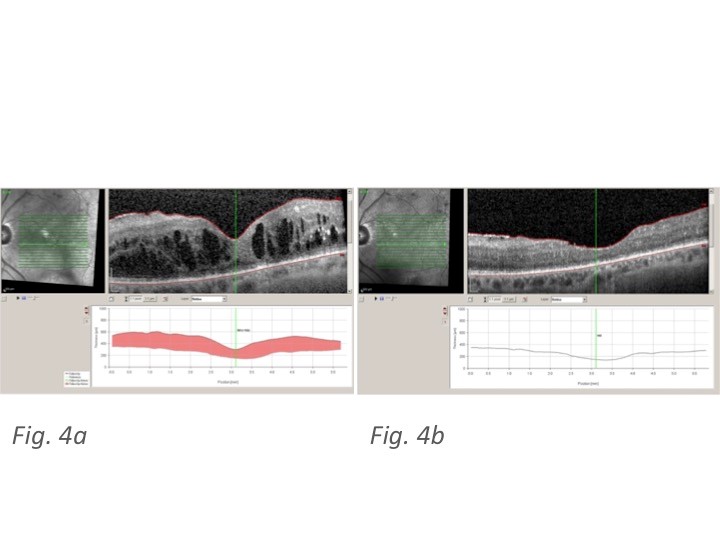
Figure 4: 4a Spectralis OCT at T0 showing increased CRT and diffuse diabetic cystoid macular edema in one of the studied eyes; 4b Spectral is OCT of the same patient at M6 showing a sharp decrease in CRT and DME following treatment
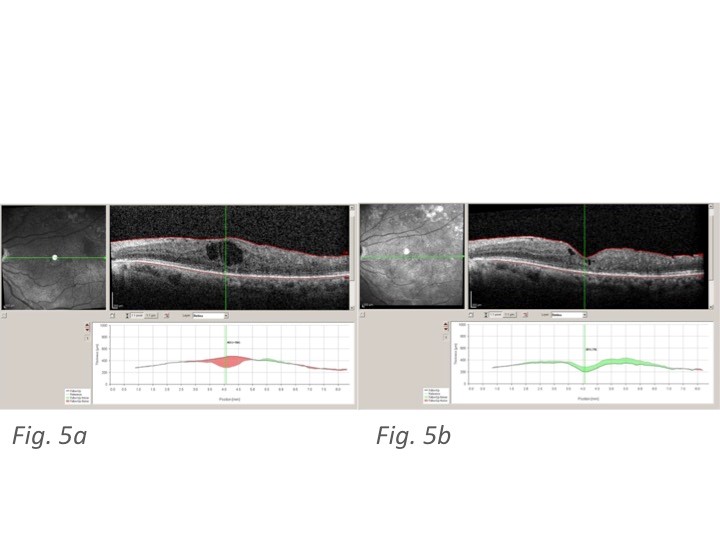
Figure 5: 5a Spectralis OCT at T0 showing increased CRT and central diabetic cystoid macular edema in another patient; 5b Spectralis OCT of the same patient at M6 showing a reduction in CRT and DME following treatment
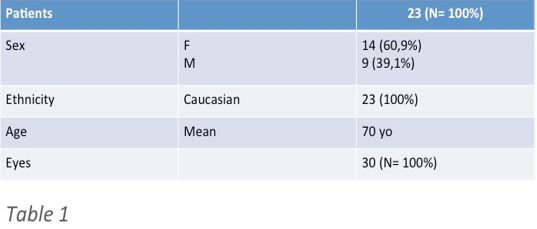
Table 1: Characteristics of patients included in the study
Citation:Alongi S, Rosa R, Murialdo U, Allegri P (2019) Intravitreal Dexamethasone Implant (Ozurdex®) Injection Preceding Phacoemulsification in Patients Affected by Cataract and Diabetic Macular Edema: Our Preliminary Results. J DiabetesMetabManag: JDMM-100001.