Journal of Obstetrics and Gynecological Problems
ISSN 2652-466X
Research Article
Breast Cancer in Men: Clinical and Pathological Analysis of 19 Cases and Review of Literature
Mension E1*, Alonso I1 and Castelo-Branco C1
1Clinic Institute of Gynecology, Obstetrics and Neonatology, Faculty of Medicine-University of Barcelona, Hospital Clinic-Institutd´Investigacions Biomèdiques August Pi iSunyer (IDIBAPS); Barcelona, Spain
*Corresponding author: Eduard Mension, Clinic Institute of Gynecology, Obstetrics and Neonatology,
Hospital Clinic-Institutd´Investigacions Biomèdiques August Pi iSunyer (IDIBAPS); Barcelona, Spain
Citation: Mension E, Alonso I, Castelo-Branco C (2020) Breast Cancer in Men: Clinical and Pathological Analysis of 19 Cases and Review of Literature. J Obstet Gynecol Probl: JOGP 100018
Received date: 10 November, 2020; Accepted date: 23 November, 2020; Published date: 30 November, 2020
Abstract
Background: Breast cancer (BC) in men is an uncommon disease lacking studies and specific recommendations on clinical guidelines focused on this group of patients. During the last years there have been an increase of cases of BC in this subgroup, and some studies suggest that differences between both genders.
Objective: To describe a cohort of male patients with BC, to evaluate its clinical characteristics, surgery approach and to review and discus critical points on male BC.
Methods: In this cross-sectional study the electronic data was collected retrospectively for the selected cases between April 2010 and June 2020. A descriptive analysis of the data and the recurrence Kaplan Meyer curve were performed.
Results: A total of 19 cases were recorded. The mean age was 65.2 years (±12.6 SD compared with 59.2 years ±16.2 SD in women), the Body Mass Index was 28.78 ± 4.9 SD, with 31.6% of cases diagnosed with gynecomastia. In all cases treated with surgery, the surgical treatment of choice was mastectomy. The average tumour diameter was 23.58mm. The immunohistochemical subtypes classification was; Luminal A-like 26.32%, Luminal B-like Her2 negative 52.63%, Luminal B-like Her2 positive 15.79%, Triple negative-like 5.26%, Her2 pure-like 0%.
Conclusion: The mean age of male BC patients is greater than that observed in women, with a higher rate of luminal histological subtypes and a more aggressive surgical approach. Due to being underrepresented or even excluded in most of the studies, today there are no-evidence to make the best approach in the management of these patients.
Keywords: Breast Cancer; Clinical Management; Immunohistochemistry; Male Breast Cancer
Introduction
Rationale: Breast cancer (BC) in men is a rare entity, occurring in approximately 1% of men and representing between 0.5 and 1% of all BC in both genders [1]. For that reason, they are unquestionably underrepresented in many of the studies on which the usual clinical practice is based. It is noteworthy that at present time we do not actually know the optimal management for this subgroup of patients with BC, which may differ from the female group. It is common to find in clinical guidelines the recommendation to treat BC in men as BC in postmenopausal women. In recent years, more attention has focused on male BC. According to a recent epidemiological study, the incidence and prevalence of this entity is increasing significantly [2].
It is known that both genders share risk factors such as aging, genetic factors and hyper-oestrogenic states such as obesity. According to literature, BC in men presents a mean age of onset 5-10 years later than the women [3]. Regarding genetic risk level, there is an increased risk of developing BC if there is involvement of a first-degree relative. Up to 20% of men suffering BC have affected first-degree relatives, compared to 7% of the general population [3]. Considering risk mutations, men present a different pattern than the female gender; being BRCA2 mutation the most common found, conferring a 6% absolute risk in life of presenting BC [4].
Previous studies document that the most frequent histological subtypes are the so-called luminal-like ones, finding rates of HER-2 positive tumours of 12.2% and 2.9% of triple negative tumours [5]. Despite being mainly the theoretically less aggressive subtypes, several studies describe a lower overall survival in BC in men compared to BC in women. Possibly, the worst prognosis in men may be influenced by the occurrence in older population, and the fact BC in men usually occur in more advanced stages [6]. Studies specifically aimed at this subpopulation are necessary to be able to effectively address the controversies that continue today without being resolved.
Objective: In this case-series and review we aim to describe the epidemiological, clinical and pathological characteristics along with the treatments received by men complaining with BC in a single centre during the last 10 years providing an update on the topic.
Material and Methods
A retrospective data collection was made of all cases of BC in men diagnosed at Hospital Clínic de Barcelona between 2010 and 2019.
Sample: Male patients diagnosed with BC between 2010 and 2019 which were in the electronic health data record of Hospital Clínic de Barcelona were included. The cases where information regarding clinical, molecular, surgery or histology variables were lacking were excluded (Figure 1).
Design: An observational, cross-sectional, descriptive analysis of the data was performed to assess the epidemiological characteristics of patients suffering from this type of cancer, alongside data on clinical variables, imaging tests performed, tumour profile, surgery, systemic treatment, pathological anatomy, and the current state of the patient.
Statistical Analysis: The descriptive analysis was performed using the "summarize" and "tabulate" tools. A recurrence descriptive analysis was described as a Kaplan Meyer graph. Statistical analysis was made using the statistical program STATA, version 15.1 (StataCorp) [7].
Results
Study Selection: A total of 19 cases of male patients suffering BC were diagnosed in our center between 2010 and 2019.
Study Findings: Clinical characteristics of the entire sample are given in table 1. Regarding epidemiological characteristics, the mean age at diagnosis was 65.2 years (± 12.6 SD) (Age distribution represented on Figure 2), the 31.58% of the patients had been diagnosed with gynecomastia, and the mean Body Mass Index (BMI) was 28.78, presenting obesity (defined as a BMI greater than 30) the 30.8% of the sample. The prevalence of recreative drugs consumers such as smoking and alcoholic habit greater than or equal to 2 units of alcohol daily were 42.1% and 30.0% respectively. No patient reported use of hormonal / anabolic therapies.
Concerning diagnostic variables, in a 41.11% of cases affected the left breast (8), in 52.63% the right breast (10), and in one case was bilateral. The mean tumour size at diagnosis was 23.58 mm and 21.05% of patients presented confirmed axillary-positive lymph node by Fine Needle Aspiration (FNA) after ultrasound suspicion of locoregional metastasis. In 18 patients (94.74%) BC was unifocal, being multifocal BC only in one case. The clinical BC stage prior to surgery were: Stage I in 20% of cases, Stage II in 46.67%, Stage III in 26.67% and Stage IV in the remaining 6.67%.
In 13 cases (68.42%), treatment started with primary surgery, and mastectomy was performed in 100% of our cases. Six of the cases underwent systemic neoadjuvant treatment (31.58%). Holding on axillary surgery, selective sentinel node biopsy (SSNB) was performed in 16 of cases (84.21%). Three of the SSNB obtained were positive), completing the following lymphadenectomy in all cases. Lymphadenectomy was performed in 5 of total cases (2 directly, 3 after SSNB result) and one patient did not receive axillary surgery.
Analyzing the 4 positive FNA cases at baseline, one did not perform treatment due to the finding of metastases, and another case received neoadjuvant therapy, and subsequent SSNB and lymphadenectomy were negative.
Focusing on the pathology, 89.47% were classified as infiltrating ductal carcinoma, 5.26% as papillary carcinoma and 5.26% as moderately differentiated hydro-carcinoma. The histological grade was classified as grade II for 94.74% of the cases, by 5.26% of grade I and no cases classified as grade III.
Examining molecular variables, 15.79% cases were found positive for HER2 receptors, 85.95% positive for estrogen receptors, 57.00% positive for progesterone receptors, and the Ki67 presented an average of 24.44%. The tumours were classified into immunohistochemical subtypes; Luminal A 26.42%, Luminal B Her2 negative 52.63%, Luminal B Her2 positive 15.79%, Triple negative 5.26%. No patients showed the subtype Her2 enriched.
The PROSIGNA genetic test was performed in 26.3% of cases, obtaining 40% of Luminal A and 60% of Luminal B. A study of mutations was carried out in 57.89% of patients, presenting one case of BRCA1 mutation, one case of BRCA2 mutation and another unique case of CHECK2 mutation.
Finally, the 15.8% of the cohort received adjuvant radiotherapy, 73.68% of the patients received chemotherapy (neoadjuvant in 31.58%) and 68.42% received tamoxifen (single systemic therapy in 23.5%). Recurrence or decease was observed in 36.6% of the patients during a 10-year follow-up. The mean survival / free of disease time was 58.89 months (Standard Error 6.02) (Figure 3).
Discussion
Male breast cancer is considered a rare disease. Due to the high incidence of BC in women, the few cases that appear in the opposite gender are dissolved or directly excluded in many of the studies that are carried out. Most of the guidelines recommend treating BC equally in both sexes, since there is no specific scientific evidence for this subpopulation.
The main risk factor described in literature is aging. It has been reported a mean age of onset of 68.4 years for BC in men [8], higher than that reported in women of about 62 years of age [9,10]. In our population during the study period, the mean age at diagnosis was greater than 65 years in men compared to a mean age of 59.3 years (± SD 16.2) in women (data not shown). The risk presented by obese patients must also be taken into account, since due to the transformation of androgens to oestrogens in adipose tissue, the lower concentration of testosterone and sex hormone-binding globulin, they usually present hyper-oestrogenic states capable of stimulate breast tissue [11]. Related to hyper-oestrogenism we find gynecomastia, a disorder that occurs in up to 70% of men over 50 years of age [12]. Studies show that the risk of atypical ductal hyperplasia is between 0.4 % to 5.4%, not finding data on the risk of BC in men suffering from gynecomastia [13].
The series of patients described in the literature agree that the luminal histological subtypes are by far the most frequently found in BC in men. In our data series we also found this characteristic in more than 80% of cases, despite being lower than the data found in articles that compare the characteristics of BC between both genders. The low prevalence of Her-2 enriched cases is striking, no cases in our series, and 0.6% of cases according to Leone et al in their recent publication [14].
When comparing the survival between BC in men to BC in females, the literature agree that the male subgroup presents a decreased overall survival. There are different theories to justify this worse prognosis. We know that males present a higher percentage of luminal-like type histological subtypes, so we would think that they may suffer from relatively less aggressive tumours. However, it is more common to find advanced stages at diagnosis, presenting up to 20% of positive axillary nodes. Some studies show that in reality the survival when comparing between men and women suffering BC in Stages III and IV is similar, but a worse prognosis in males have been found in Stages I and II at diagnosis compared to females [14].
In our series we describe that up to 33% of cases are diagnosed in advanced stages, with 21% of N1 cases being diagnosed. To date, 36% of cases have had a recurrence or died on a 10-year-follow up after diagnosing, suggesting a worse prognosis compared to female patients diagnosed with BC [15]. This increased risk does not seem to be due to the diagnosis in higher stages, nor to histological subtypes with a worse prognosis [16]. Larger studies are needed, specifically aimed at this population, to study if there are biological factors that grant this increased risk per se.
Finally, it is worth discussing whether we could reduce the diagnosis in advanced stages with better diagnostic tools, or information for this population group. We do not have data, but it could be that we are dealing with cases which had a late diagnosis due to the low suspicion of BC in men, which would mean that cases of breast tumours in men were not prioritized.
According to the literature and our data, due to a smaller breast size in men, the most common surgery is mastectomy (100% of the cases in our series) [17]. There is a new trend to try to be less aggressive if the tumour size allows us to try to maintain more favourable cosmetic results, and even offer reconstruction with oncoplastic techniques [18].
The exposed paper present various limitations. Given that BC is a rare disease in males, it is difficult to obtain cohorts of patients with large sizes, so the N of patients obtained may be a limitation when drawing conclusions from the data.
When performing a cross-sectional and retrospective study, any correlation of variables found may be due to the biases inherent in this type of study.
Conclusion
We conclude that in our case series we have a mean age of male patients greater than in females. Men with BC have in general a worse prognosis despite presenting less aggressive histological subtypes. This may be due to a possible diagnostic delay, finding up to 33% of cases with advanced stages. Moreover, BC in men is treated using a more aggressive surgical approach, presenting a 100% of cases treated with mastectomy in our series.
Due to being underrepresented or even excluded in most studies regarding the treatment of breast cancer, today we do not have clear evidence to make the best clinical decisions in the subgroup of men with BC. More studies directed at this population are required to study possible unknown biological risks, in addition to studies to assess de-escalation of the type of surgery performed in these patients, since despite presenting small tumours in some cases, the usual treatment continues to be mastectomy.
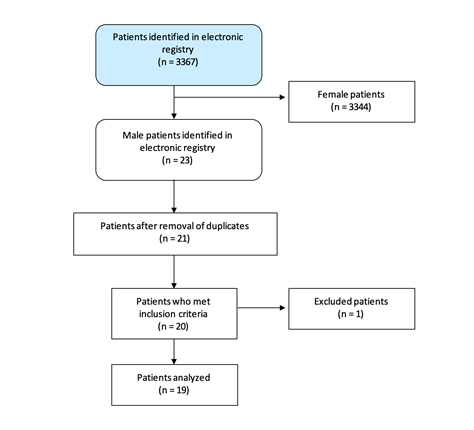
Figure 1: Flowchart diagram of the study.
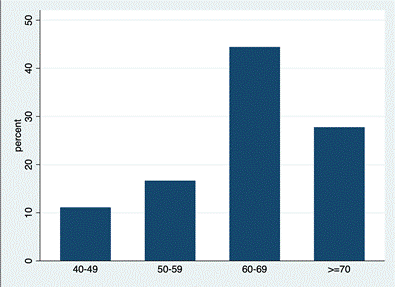
Figure 2: Age at diagnosis for the 19 cases reported.
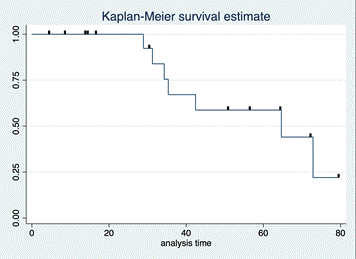
Figure 3: Kaplan Meyer Recurrence curve estimate in months.
|
Epidemiologic Variables |
Mean |
St. Dev. |
Min |
Max |
% (n) |
|
Age |
65,21 |
12,77 |
42,87 |
91,38 |
- |
|
BMI |
28,78 |
4,96 |
22,60 |
38,42 |
- |
|
Alcohol |
- |
- |
- |
- |
31,58 (6) |
|
Tobacco |
- |
- |
- |
- |
42,11 (8) |
|
Histology |
- |
- |
- |
- |
|
|
IDC |
- |
- |
- |
- |
89,47 (17) |
|
PC |
- |
- |
- |
- |
5,26 (1) |
|
MDHC |
- |
- |
- |
- |
5,26 (1) |
|
Molecular |
|||||
|
ER |
85,95 |
21,89 |
0 |
100 |
- |
|
PR |
57,00 |
36,78 |
0 |
100 |
- |
|
Ki67 |
24,44 |
14,11 |
5 |
60 |
- |
|
HER2 positive |
- |
- |
- |
- |
15,79 (3) |
|
Immunohistology subtypes |
|||||
|
Luminal A like |
- |
- |
- |
- |
26,32 (5) |
|
Luminal B HER2 positive like |
- |
- |
- |
- |
15,79 (3) |
|
Luminal B HER2 negative like |
- |
- |
- |
- |
52,63 (10) |
|
HER2 enriched like |
- |
- |
- |
- |
0 |
|
TN like |
- |
- |
- |
- |
5,26 (1) |
Table 1: Descriptive analysis of study variables. St. Dev.: Standard Deviation, BMI: Body Mass Index, IDC: infiltrating ductal carcinoma, PC: papillary carcinoma, MDHC: moderately differentiated hydro-carcinoma, ER: estrogen receptors, PR: progesterone receptors, TN: Triple negative.
Citation: Mension E, Alonso I, Castelo-Branco C (2020) Breast Cancer in Men: Clinical and Pathological Analysis of 19 Cases and Review of Literature. J Obstet Gynecol Probl: JOGP 100018