Anders Overgaard, Henning Bliddal*, Marius Henriksen
The Parker Institute, Copenhagen University Hospital Fredriksberg, Copenhagen, Denmark
*Corresponding author: Henning Bliddal, The Parker Institute, Copenhagen University Hospital Fredriksberg, Copenhagen, Denmark, Tel: +4538164155.
Citation: Overgaard A, Bliddal H, Henriksen M (2019) Safety of Intra-Articular Polyacrylamide Hydrogel for the Treatment of Knee Osteoarthritis Symptoms: A Retrospective Case Series. Clin Ortho Adv Res J: COARJ-100001
Received Date: 20 December, 2018; Accepted Date: 31 December, 2018; Published Date: 22 January, 2019
1. Abstract
Objective: To assess the safety intra-articular (IA) polyacrylamide gel (PAAG) treatment for knee OA symptoms.
Methods: A case series of patients was studied, who had been treated with intra-articular injection of 2.5 % polyacrylamide hydrogel between March 2010 and February 2017. Safety assessments were performed at follow-up and included patient-reported adverse events (AEs), medical records review, radiograms, and a clinical examination of the treated knee. For patients, who had received knee arthroplasty surgery since treatment, the surgical records were reviewed for any unexpected findings.
Results: Of 128 invited patients, 91 (71%) participated. The majority (n=66, 73%) of the patients had not experienced adverse events or discomfort in relation to receiving single or multiple treatments with PAAG. Fifteen patients recalled a sensation of distention of the knee joint after the treatment, while in 14 (93%) this was passing within days to weeks. Ten patients (67%) described this sensation as mild. Two patients sought medical assistance after treatment with IA PAAG due to aggravated pain and effusion, likely to be related to the treatment. These patients were treated medically, resulting in no lasting adverse effect. Neither IA infections nor allergic reactions were reported.
Conclusion: No significant incidence was noted of serious adverse events related to IA treatment with a proprietary 2.5 % cross-linked polyacrylamide gel.
2. Introduction
Osteoarthritis (OA) is a very common chronic disease characterized by pain and physical disability [1]. More than 10% of persons 55 years of age or older have symptomatic osteoarthritis (OA), primarily involving the knees [2]. Knee OA is associated with significant impairments and limitations in mobility, self-care, and housekeeping activities, as well as limited participation in societal and recreational activities. Altogether this leads to reduced quality of life and dependency on other persons or aids.
Although OA is a very common disease with serious individual and societal consequences, a cure has not yet been identified and management focuses on symptom control and relief of pain. Even for this purpose, non-surgical treatments with long-lasting effects have not been found. Thus, there is an unmet need for new therapeutic agents and devices. A recently proposed option is polyacrylamide hydrogels (PAAG). PAAGs are non-toxic [3-5], non- degradable synthetic products, that are frequently used for soft tissue augmentation [6-11]. IA injection of PAAG in rabbit and horse joints results in a stable, long-lasting sub-synovial layer of gel traversed with thin strands of connective tissue [12]. In horses with OA IA PAAG significantly reduces lameness and joint effusion, without adverse effects [13]. Thus, IA injection of PAAG may be suggested for treatment of knee OA in humans as well, and preliminary experience has been gathered. However, before launching the PAAG treatment at a larger scale, safety needs to be evaluated.
The present retrospective case series aims at investigating the incidence and nature of any adverse events in a group of knee OA patients, who have been treated with IA PAAG.
3. Patients and methods
The study is a retrospective assessment of the safety of intra-articular (IA) PAAG given to patients with knee OA as symptomatic treatment. PAAG is an approved product for soft tissue augmentation, but the patients in this study had received the compound as an off-label treatment of their knee OA symptoms at a rheumatology and sports medicine clinic in Denmark from March 2010 and February 2017. Inclusion criteria were painful knee(s) with confirmed radiological signs of OA. Exclusion criteria were contraindications to injection, e.g. skin defects or signs of infection, inflammatory arthritis, immunosuppressive treatment, and wide spread pain.
During this period, 128 patients had received PAAG on one or more occasions for OA symptoms in the knee. All patients had accepted recall for safety registration and this was performed at the outpatient osteoarthritis clinic at Frederiksberg Hospital in Denmark based on interviews, clinical examinations, and review of medical records. Patients, who were unable to speak Danish or who were not able to remember the treatment due to dementia, were not included.
a. PAAG treatment
PAAG is a non-toxic and non-immunogenic biocompatible polymer gel [3-5] consisting of 97.5% sterile water and 2.5% cross-linked polyacrylamide produced by Contura International A/S. The tested treatment regimen encompassed IA treatment of 3 ml PAAG injected into the knee joint cavity under ultrasound guidance. The IA treatment was accompanied by a single dose oral antibiotic (azithromycin 500 mg and moxifloxacin 400 mg) given on the morning of the treatment.
b. Safety assessment
The patients were referred to the osteoarthritis outpatient clinic for the safety follow-up. If the patients had no possibility of showing up or declined a clinical examination, a telephone interview was conducted.
Each participant was interviewed and examined for evidence of adverse events (AE) and serious adverse events (SAE), retrospectively. Medical records were reviewed, and an x-ray was taken to evaluate current radiological OA classification. Knee joint range of motion (ROM) was evaluated clinically to assess any significant reduction in ROM and the examiner assessed if any decreased ROM was attributable to bony or soft tissue changes.
All patients were asked to describe any pain and mobility limitations, skin conditions, systemic events, whether or not knee related, that they experienced in relation to the PAAG treatment. If any AEs or discomforts were described, the patient was asked to describe duration (“Days”, “Weeks”, “Months”) and severity (“Mild”, “Moderate”, “Severe”).
To assess if any SAEs had occurred, all patients were asked if they experienced any events in relation to the treatment that caused them to seek medical assistance or caused hospitalization. Any such reports were pursued further by contacting the patients’ general practitioner and reviewing medical records. All AEs were recorded, including the approximate date of onset, description, severity, duration and outcome, relationship of the AE to PAAG treatment, and any action(s) taken.
Other treatments and concomitant illnesses were taken into account.
c. Post PAAG treatment knee surgery
For patients who had undergone surgery after the treatment with PAAG, the surgical reports were retrieved and reviewed for descriptions of any abnormal findings during the surgery (i.e. unexpected findings e.g. foreign bodies, granulomas, excess fibrosis, discoloration etc.).
4. Results
128 domestic patients were identified in the files of the Rheumatology and Sports Clinic. All received a letter explaining the study as a safety assessment and, in case of no response, contact was attempted by telephone. 91 responded to the invitation and represent the current retrospective safety population, SP (Figure 1). Of the 91 patients in the SP, 74 were examined at the outpatient clinic, and 17 patients participated by way of a telephone interview. There were 46 (51%) females and 45 (49%) males. The average age of the patients was 70 years (SD 13 years, range 38 to 94). The safety assessment was done on average 24 months (range 4 to 87 months) after the first treatment with PAAG. Reasons for non-inclusion in the safety population were: Not responding to the invitation (n=14) and declining the invitation (n=13). Other reasons were death (n=3), do not remember treatment (n=2), dementia (n=1), do not speak Danish (n=1), and lastly the patients that did not show at the appointed time at the outpatient clinic (n=3) (Appendix, figure 1).
a. Safety assessment
The majority of the patients (n=66; 73%) reported no discomfort or untoward events in relation to the treatment of their knee OA with IA PAAG. Twenty-five patients (27%) reported adverse events/discomforts. These 25 patients reported 41 items of discomfort. Most patients (n=13) reported only 1 item and 4 patients reported 3 or more items. Patients reported mostly a sensation of distention (n=15) and worsening of pain from target knee (n=7). To a lesser extent, the patients reported other pain in the leg and muscle discomforts and soreness (n=6). There were 4 reports of a reduced range of motion and 2 of stiffness (Table 1).
Seven neurological symptoms such as a prickling sensation, numbness, burning or feeling of cold or heat were reported (Table 2).
Of the 7 patients reporting pain from target knee 4 reported the pain to be severe. All patients reporting pain described the duration of the event to be resolved within weeks. The patients reporting distention of the knee mostly (n=10) described this to be mild and 14 of the 15 patients reported the distention to resolve within weeks, while in one case the patient described the effect to last more than a month.
There were 5 reports of discomforts lasting more than a month from initial treatment (Distention, reduced range of motion, stiffness, cold sensation, and prickling sensation). When interviewed none of the patients described lasting symptoms. Patient-reported severity and duration is summarized in (Tables 3 and 4).
Two patients reported to have sought medical assistance at the treating clinic because of knee pain after the initial treatment. One was treated with analgesics and was in remission after a week. The other had intra-articular effusion and was treated with recurrent arthrocentesis, one and three weeks after the initial treatment. No lasting reactions were reported.
From the clinical examinations of the 74 treated knees, the investigator found no signs of reduced knee ROM other than what is expected in a population of knee OA patients.
No intra-articular infections or allergic reactions were reported in this retrospective study.
b. Post PAAG treatment knee surgery
15 (16%) of the 92 participants had, since initial treatment with PAAG, received arthroplasty surgery for knee OA of the treated knee. Of these, it was possible to retrieve 14 surgical reports, the missing report was held by a private clinic and could not be obtained before data analysis had begun. From the pre-operative examinations, all patients were described as having ROM as expected by the status of their knee OA. There were no unexpected descriptions of abnormalities that could be associated with the prior treatment with PAAG in the surgical reports. None of the reports mentioned the presence of unidentified foreign bodies, discolorations, or macroscopical signs of fibrosis. Of the 15 patients with arthroplasties, 13 were examined at the osteoarthritis outpatient clinic by the investigators. Of these patients, none reported dissatisfaction with the outcome of the surgery and the arthroplasties were well functioning. The average time from first treatment with PAAG to surgery was 19 months (range 5 to 58 months).
5. Discussion
This study presents real-life data of a long-term follow-up after IA administration of PAAG between 4 months and 7 years prior to safety assessment. Few, tolerable, and short-lived discomforts were related to the PAAG treatment.
Importantly, we found no indications of disadvantages or side effects that are dissimilar to those known from other available intra-articular treatment modalities for knee OA on the market today. As with other IA treatments for OA (e.g. corticosteroid), some patients (27%) reported an initial aggravation of the OA symptoms. However, these were reported to resolve quickly, similar to other injection therapies. Furthermore, there were no reports of allergic or systemic reactions to the PAAG. These findings are in accordance with the experience of PAAG given to animals showing few adverse events and also with human use of PAAG for soft tissue augmentation [9-13].
The absence of macroscopical anomalies in the surgery reports is in line with the previous histological reports of animal knees treated with PAAG [12], which showed an integration or sub-lining of the PAAG of the synovium, with the sub-synovial zone retaining its thickness.
Related to the treatment, 15 patients described post-injection sensations of distention of the knee. This may be speculated to be due to an elevated intra-articular pressure and distention of the joint capsule, or a synovial reaction. However, based on this retrospective case series the cause of this sensation is not clear. Nevertheless, most patients (93%) described the sensation as transient and resolving over days to weeks. Intra-articular injection therapies are normally associated with mild injection pain of short duration and short lived effusion is common [14-16].
6. Limitations and strengths
This safety assessment has several inherent weaknesses - especially the recall bias due to the retrospective nature of the adverse event reporting is a limitation. Some patients described problems in recollecting events in relation to treatment or even the treatment. However, we are confident that patients would recall if an adverse event had a significant impact on their lives even several years back. The relatively few reported significant adverse events are supported by the generally positive patient satisfaction that further supports the beneficial safety profile of IA PAAG treatment.
The strength of the study is the rather large and comprehensive material, which allowed us to give a qualified estimate of the possible adverse events based on real-life data. The Danish Hospital system has a well-functioning filing system and all hospital admissions are registered. Also, the patients’ GPs were compliant with supplying us with data from their files of events in the aftermath of PAAG treatment. Thus, it may be claimed that all serious adverse events have been reported as well as most other events requiring a doctor’s intervention.
Based on the present study, we suggest that knee OA treated with the application of PAAG is safe and may represent a relevant option for further investigation. Although, the current results need confirmation in prospective studies of both efficacy and safety, they provide useful information about the safety of this new product and open new perspectives in the area of non-surgical OA management.
7. Conclusion
This retrospective case series of patient-reported safety, clinical examinations, and medical record reviews, found no significant incidence of adverse events or serious adverse events related to the intra-articular treatment with a proprietary 2.5 % cross-linked polyacrylamide gel for the relief of knee osteoarthritis pain and disability.
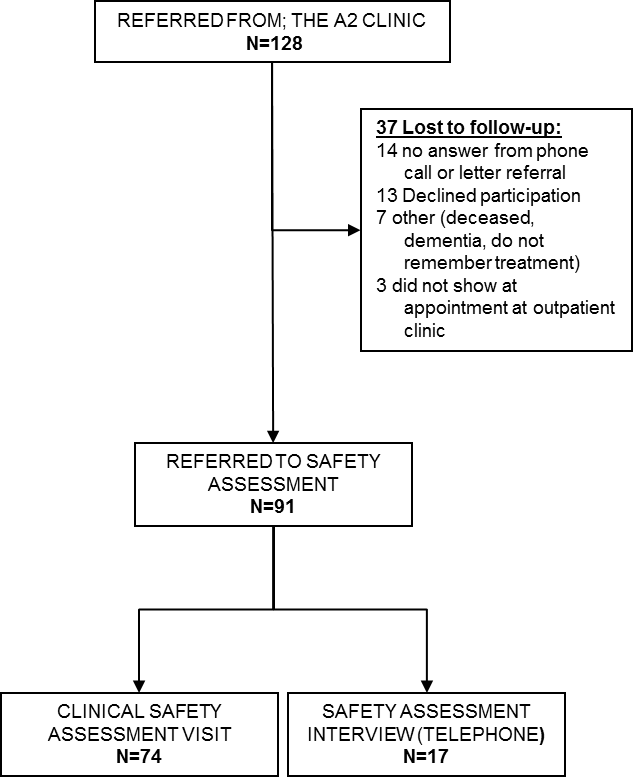
Figure 1: Flow chart
|
Demographics |
Telephone |
Seen at the outpatient-clinic |
|---|---|---|
|
|
Mean (SD) |
Range (SD) |
|
n |
17 |
74 |
|
Age, years |
73 (12) |
70 (10) |
|
Gender (female), n |
11 |
35 |
|
Followup (months) |
18 (12) |
25 (19) |
|
Kellgren-Lawrence score, n (%) * |
|
1 = 3 (5%) 2 = 15 (25%) 3 = 23 (38 %) 4 = 20 (33 %) |
|
* n=61 , 13 patients had undergone surgery before follow-up |
||
|
Reported adverse events or discomfort |
Patients describing no adverse events or discomfort |
|---|---|
|
N = 25 (27%) |
N = 66 (73%) |
Table 1: Number (proportions) of patients retrospectively reporting adverse events/discomfort in relation to treatment with PAAG
|
Adverse event registered |
No. |
Proportion |
|---|---|---|
|
Pain from target knee |
7 |
17.1% |
|
Other pain from extremity |
1 |
2.4% |
|
Muscle discomfort/pain |
2 |
4.9% |
|
Soreness |
3 |
7.3% |
|
Burning sensation |
1 |
2.4% |
|
Sensation of distension |
15 |
36.6% |
|
Skin or joint pricking sensation |
3 |
7.3% |
|
Numbness |
1 |
2.4% |
|
Cold sensation |
1 |
2.4% |
|
Heat sensation |
1 |
2.4% |
|
Reduced range of motion |
4 |
9.8% |
|
Stiffness |
2 |
4.9% |
|
Total |
41 |
100% |
Table 2: Patient reported events
|
|
n |
Proportion |
Severity |
n |
|---|---|---|---|---|
|
Pain from target knee |
7 |
17.1% |
Mild |
2 |
|
|
Moderate |
1 |
||
|
|
Severe |
4 |
||
|
Other pain from extremity |
1 |
2.4% |
Mild |
|
|
|
Moderate |
|||
|
|
Severe |
1 |
||
|
Muscle discomfort/pain |
2 |
4.9% |
Mild |
2 |
|
|
Moderate |
|||
|
|
Severe |
|||
|
Soreness |
3 |
7.3% |
Mild |
3 |
|
|
Moderate |
|||
|
|
Severe |
|||
|
Burning sensation |
1 |
2.4% |
Mild |
1 |
|
|
Moderate |
|||
|
|
Severe |
|||
|
Sensation of distension |
15 |
36.6% |
Mild |
10 |
|
|
Moderate |
2 |
||
|
|
Severe |
3 |
||
|
Skin or joint pricking sensation |
3 |
7.3% |
Mild |
1 |
|
|
Moderate |
1 |
||
|
|
Severe |
1 |
||
|
Numbness |
1 |
2.4% |
Mild |
1 |
|
|
Moderate |
|||
|
|
Severe |
|||
|
Cold sensation |
1 |
2.4% |
Mild |
1 |
|
|
Moderate |
|||
|
|
Severe |
|||
|
Heat sensation |
1 |
2.4% |
Mild |
1 |
|
|
Moderate |
|||
|
|
Severe |
|||
|
Reduced range of motion |
4 |
9.8% |
Mild |
1 |
|
|
Moderate |
2 |
||
|
|
Severe |
1 |
||
|
Stiffness |
2 |
4.9% |
Mild |
2 |
|
|
Moderate |
|||
|
|
Severe |
Table 3: Patient-reported severity of the reported events
|
n |
Proportion |
Duration |
n |
|
|---|---|---|---|---|
|
Pain from target knee |
7 |
17.1% |
Days |
3 |
|
Weeks |
4 |
|||
|
Months |
||||
|
Other pain from extremity |
1 |
2.4% |
Days |
1 |
|
Weeks |
||||
|
Months |
||||
|
Muscle discomfort/pain |
2 |
4.9% |
Days |
2 |
|
|
Weeks |
|||
|
|
Months |
|||
|
Sore |
3 |
7.3% |
Days |
2 |
|
|
Weeks |
1 |
||
|
|
Months |
|||
|
Burning sensation |
1 |
2.4% |
Days |
1 |
|
|
Weeks |
|||
|
|
Months |
|||
|
Sensation of distension |
15 |
36.6% |
Days |
6 |
|
|
Weeks |
8 |
||
|
|
Months |
1 |
||
|
Skin or joint pricking sensation |
3 |
7.3% |
Days |
1 |
|
|
Weeks |
1 |
||
|
|
Months |
1 |
||
|
Numbness |
1 |
2.4% |
Days |
1 |
|
|
Weeks |
|||
|
|
Months |
|||
|
Cold sensation |
1 |
2.4% |
Days |
|
|
|
Weeks |
|||
|
|
Months |
1 |
||
|
Heat sensation |
1 |
2.4% |
Days |
|
|
|
Weeks |
1 |
||
|
|
Months |
|||
|
Reduced range of motion |
4 |
9.8% |
Days |
1 |
|
|
Weeks |
2 |
||
|
|
Months |
1 |
||
|
Stiffness |
2 |
4.9% |
Days |
|
|
|
Weeks |
1 |
||
|
|
Months |
1 |
Table 4: Self-reported duration of reported AE or discomfort
Citation: Overgaard A, Bliddal H, Henriksen M (2019) Safety of Intra-Articular Polyacrylamide Hydrogel for the Treatment of Knee Osteoarthritis Symptoms: A Retrospective Case Series. Clin Ortho Adv Res J: COARJ-100001