Annals of Medical & Surgical Case Reports
(ISSN 2652-4414)
Case Report
Rapid Dramatic Response to Alpelisib Plus Fulvestrant in a Heavily Pretreated Patient with PIK3CA-Mutated Metastatic Breast Cancer and Pulmonary Visceral Crisis
Moukadem HA1, Amhaz G1, Shihab L2 and El Saghir NS1*
1Department of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon
2Department of Nursing, American University of Beirut Medical Center, Beirut, Lebanon
*Corresponding author: Nagi S. El Saghir, MD, FACP, FASCO, Department of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon
Citation : Moukadem HA, Amhaz G, Shihab L, El Saghir NS (2020) Rapid Dramatic Response to Alpelisib Plus Fulvestrant in a Heavily Pretreated Patient with PIK3CA-Mutated Metastatic Breast Cancer and Pulmonary Visceral Crisis. Ann Med &Surg Case Rep: AMSCR-100071
Received date : 23 October, 2020; Accepted date : 28 October, 2020; Published date : 04 November, 2020
Abstract
We present in this report the case of a patient with hormone receptor (HR)-positive HER-2/neu negative metastatic breast cancer (MBC) with lymphangitic spread in pulmonary visceral crisis and with a positive phosphatidylinositol-4,5-bisphosphonate 3-kinase cancer (PIK3CA) gene mutation treated with PI3K inhibitor (PI3Ki) alpelisib in combination with fulvestrant after multiple lines of therapy. The patient had a rapid and dramatic response to treatment and was taken off oxygen therapy within a few days and remained progression free for 9 months. This is the first report of a rapid, early and sustained response to alpelisib plus fulvestrant in a patient with HR-positive HER2-negative MBC in visceral crisis treated successfully with alpelisib after having received 12 lines of hormonal, targeted and cytotoxic therapies, that included mTOR and CDK 4/6 inhibitors over a 12 years period. This report illustrates that PIK3CA mutation can be a driver of the malignancy even in late stages of disease, as well as the importance of studying the mechanisms of resistance to alpelisib.
Keywords: Alpelisib; PIK3CA mutation; Hormonal therapy; Metastatic breast cancer; Visceral crisis
Introduction
The activating mutation of PIK3CA gene are present in more than 30% of early [1-4] and metastatic5 breast cancer biopsies, up to 45% and 29% in luminal A and luminal B subtypes respectively. The association between the PIK3CA mutation and prognosis is distinguished between early and metastatic HR-positive HER-2/neu negative breast cancer. In early breast cancer, the PIK3CA mutation is associated with a better recurrence-free survival [6] and a better disease-free survival [7]; while recent molecular profiling from MBC data showed that a PIK3CA mutation would indicate resistance to chemotherapy and a poor outcome [8]. In a systemic review of prognostic and predictive value of PIK3CA mutations of articles published from 1993 till 2019, the presence of PIK3CA mutations may be associated with worse clinical outcomes in patients with HR positive HER-2/neu negative MBC [9]. The PI3K/Akt/mTOR pathway has been described as a regulatory mechanism of secondary endocrine resistance in HR positive breast cancer [10]; thus, clinical outcomes may be improved through combining a PI3Ki and endocrine therapies among this population [9].
PI3Ks are a family of lipid kinases that are divided into classes and subclasses; class IA PI3K heterodimers contain a p110 catalytic unit and a p85 regulatory unit [3]. The most common aberration in the PI3K pathway in breast cancer is inducing hyperactivation of the alpha isoform (p110α) with two hot-spot regions for mutations in exon 9 and 202. Alpelisib is an isoform-specific PIK3i that inhibits the class I p110α and is around 50 times stronger than other isoforms [11]. Alpelisib is approved by Food and Drug Administration for HR positive HER-2/neu negative PIK3CA-mutated MBC after the phase III trial SOLAR-1 study that evaluated the efficacy and safety of alpelisib with fulvestrant in patients previously treated with endocrine therapy. The improvement in progression free survival (PFS) was 11 months compared to 5.7 months in the control group in the PIK3CA mutant cohort and not in the PIK3CA wild type cohort1. The overall response defined by complete or partial response was observed in 35% of patients; while clinical benefit is seen in 57% [1]. In this study, grade 3-4 hyperglycemia occurred in 36.6% of patients and permanent discontinuation due to hyperglycemia was 6.3% [1]. Thus, the management of the safety profile is essential for continuation of treatment in case of response to therapy.
Case presentation
We report the case of a 50-year-old female who was diagnosed with de novo estrogen receptor positive progesterone receptor positive HER-2/neu negative infiltrating ductal carcinoma of the left breast with metastasis to lymph nodes, lungs and bones at the age of 38. Over a 12 years period, the patient was treated subsequently with multiple lines of single agents or combination therapies that included docetaxel with capecitabine, bevacizumab, tamoxifen, FAC (5FU, Adriamycin, and cyclophosphamide), weekly paclitaxel and gemcitabine, vinorelbine, goserelin, everolimus with letrozole, CMF (cyclophosphamide, methotrexate, and 5FU), palbociclib and fulvestrant, eribulin, liposomal doxorubicin, bilateral oophorectomy, metronomic chemotherapy using oral vinorelbine and capecitabine, and carboplatin with paclitaxel. Patient also received pelvic bone irradiation in 2018 and radiotherapy to hip in 2019.
In September 2019, she presented to AUBMC Emergency Room with severe shortness of breath. She had dyspnea, pleuritic chest pain and desaturation requiring oxygen through nasal cannula at rest. At that time, the patient was on treatment with everolimus and exemestane for 9 months. Chest X-ray and Chest CT scan showed increased reticulonodular infiltrates consistent with diffuse and bilateral pulmonary metastasis, lymphangitic spread, and a small left sided pleural effusion (Figure 1).
Results of Next Generation Sequencing by Foundation One came back showing the presence of PIK3CA mutation in tumor cells. Therefore we stopped everolimus and exemestane and started therapy with alpelisib at a dose of 300 mg daily, in combination with fulvestrant. After only four days on alpelisib, the patient had significant clinical improvement in chest discomfort and dyspnea. She quickly became oxygen-independent and fully ambulatory. The patient remained well on alpelisib combination for 9 months. She developed severe hyperglycemia requiring insulin supplementation in addition to oral hypoglycemic agent metformin. Tumor marker Ca15.3 was 925 before treatment of alpelisib and decreased down to 471 at 3 months and 183 at 6 months. Evaluation by radiologic imaging PET CT after 3 months of treatment revealed a significant response alpelisib and fulvestrant with interval decrease in size and activity of pulmonary and pleural nodules, decrease in left pleural effusion and resolution of right pleural effusion, decrease in left breast nodules, decrease in the retroperitoneal lymphadenopathy with increased sclerosis and decreased uptake in bone lesions (Figure 2). In May 2020, ten months after the start of alpelisib combination, the patient had shortness of breath, Ca15.3 rose up to 326 and PET CT scan showed overall progression of disease in the breast, lymph nodes, lungs, liver, and bones. We concluded that the patient has a relapse, 9 months after alpelisib plus fulvestrant combination therapy.
Figure 2A: PET/CT Scan before alpelisib combination therapy shows extensive pulmonary metastasis with high SUV hypermetabolic activity.
Figure 2B: PET/CT Scan after 3 months of alpelisib and fulvestrant shows significant improvement.
Discussion
Over the last 5 years, major advances emerged in the understanding of pathways of proliferation and mechanisms of resistance in patients with HR-positive HER2-negative MBC. Understanding pathogenesis helped researchers identify targets for therapy including CDK4/6 and PI3K/Akt/mTOR pathway. Alpelisib, a selective PI3Ki, showed an overall response rate of 35% and PFS was prolonged by 5 months in patients who had prior hormonal therapy in the Solar-1 phase 3 clinical trial1. The major toxicity profile included hyperglycemia (63.7%), diarrhea (57.7%), nausea (44.7%), decreased appetite (35.6%) and rash (35.6%)1. In the present reported case, the patient had severe hyperglycemia and required insulin supplementation in addition to oral hypoglycemics.
In Solar-1, more than half of the patients in the PIK3CA mutant cohort received alpelisib in the first line of treatment for advanced disease (52.1%), while 46.7% of the patients received alpelisib as second line treatment for MBC. In the cohort without PIK3CA mutation, 61.7% of patients had alpelisib in the first line and 36.5% in the second line1. In Solar-1, only 7% of patients had any CDK 4/6 inhibitor and up to 60% had previous chemotherapy in the adjuvant and neo-adjuvant setting. Our patient responded very well to alpelisib after 9 lines of prior chemotherapy including taxanes and anthracyclines, and 3 lines of hormonal therapy that included tamoxifen, aromatase inhibitors, fulvestrant, mTOR and CDK4/6 inhibitors. We note that in June 2020, an open-label non-comparative phase II study (BYLieve study) was presented at ASCO annual meeting with a partial response rate of 17% [12].
In Solar-1 trial , 55% of patients had any visceral site metastasis1. No visceral crisis was included in the 169 patients treated with alpelisib and fulvestrant in the cohort of PI3KCA-mutated cancers. Most guidelines, including Advanced Breast Cancer (ABC) International Consensus Guidelines recommend the use of chemotherapy for such patients [13].
Our reported patient is the first case of refractory metastatic breast cancer in pulmonary visceral crisis, after 12 lines of chemotherapy and hormonal therapy that included CDK4/6 inhibitors and fulvestrant, who had a very rapid response to alpelisib plus fulvestrant that lasted for 9 months.
The best sequencing strategies of hormonal therapy and CDK 4/6, mTOR, and PI3K inhibitors remains unknown. ABC5 guidelines state that effectiveness of PIK3CA inhibitors in patients who previously received a CDK 4/6 inhibitor remains unknown since only about 7% of patients in SOLAR-1 study had previously been treated with a CDK4/6 inhibitor [13].
In conclusion, this is the first case of a patient with PIK3CA-mutated HR-positive HER2-negative refractory chemotherapy-resistant (9 lines of cytotoxic chemotherapy) hormonal therapy-resistant (3 lines of hormonal therapy that included tamoxifen aromatase inhibitors, goserelin, fulvestrant, mTOR inhibitors, CDK4/6 inhibitors bilateral oophorectomy) metastatic breast cancer in pulmonary visceral crisis, with prior treatment with mTOR inhibitors, CDK4/6 inhibitors and fulvestrant, treated with PI3Kc alpha-specific inhibitor alpelisib and fulvestrant and had an excellent, rapid and durable response. This case report confirms that PIK3CA mutation can remain a driver of malignancy in refractory breast cancer, and that alpelisib combination deserves to be studied in later lines of therapy for patients with PIK3CA mutated MBC.
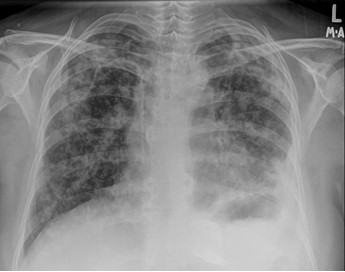
Figure 1: Chest X-ray showing extensive pulmonary metastasis and lymphangitic spread (prior to initiation of alpelisib and fulvestrant therapy).
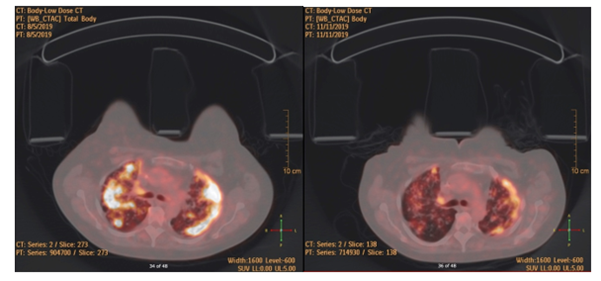
Figure 2: PET/CT scan images before and after alpelisib.
Citation : Moukadem HA, Amhaz G, Shihab L, El Saghir NS (2020) Rapid Dramatic Response to Alpelisib Plus Fulvestrant in a Heavily Pretreated Patient with PIK3CA-Mutated Metastatic Breast Cancer and Pulmonary Visceral Crisis. Ann Med &Surg Case Rep: AMSCR-100071