Journal of Urology and Renal Problems
(Volume 02; Issue 02)
Research Article
The Prediction Value of AntithrombinIII on the of Contrast-Induced Nephropathy in Coronary Artery Disease Patients Undergoing Percutaneous Coronary Intervention
Jinyang Lu1, Yanhu Sun1, Yuhan Li1, Di Zheng1,2, Quan Zhang1,2,Wenhua Li1,2*
1Institute of Cardiovascular Disease, Xuzhou Medical University, China
2Department of Cardiology, Affiliated Hospital of Xuzhou Medical University, China
*Corresponding author: Prof. Wenhua Li, Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, 99 Huaihai West Road, Xuzhou, Jiangsu 221002, P.R. China.
Citation: Lu J, Sun Y, Li Y, Zheng D, Zhang Q, et al. (2020) The Prediction Value of Antithrombin III on the of Contrast-Induced Nephropathy in Coronary Artery Disease Patients Undergoing Percutaneous Coronary Intervention. J Urol Ren Probl: JURP-100011.
Received Date: 21 August, 2020; Accepted Date: 25 August, 2020; Published Date: 30 August, 2020
Abstract
To evaluate the significance of early prediction of AntithrombinIII (AT-III) for Contrast- Induced Nephropathy (CIN) in coronary artery disease patients undergoing Percutaneous Coronary Intervention (PCI). A total of 300 patients were enrolled. Basic information of each case were well recorded. Levels of SCr were measured at baseline (before PCI) and within 48 to 72 hours after the administration of a contrast agent. CIN was defined as a 0.5 mg/dL or 25% increase in SCr levels 48 to 72 hours following exposure to contrast media. The incidence of CIN was 13.67%(41/300). The levels of AT-III in the CIN group were significantly increased compared with the non-CIN group (P<0.05). With the increasing of AT-III levels, the differences between SCr showed a decreasing trend before and after PCI (P<0.05). Logistic regression analysis presented that AT-III was an independent protective factor for CIN. The Area Underthe Curve (AUC) of AT-III was 0.771 for the prediction of CIN, and the best cut-off value was 89.5% with sensitivity, specificity, positive predictive value, and negative predictive value of 80.5%, 72.2%, 31.4%, 95.9%, respectively. The levels of AT-III might be a useful predictor of CIN.
Keywords: Antithrombin III; Contrast-Induced Nephropathy; Percutaneous Coronary Intervention
Introduction
Contrast-Induced Nephropathy (CIN) is an iatrogenic kidney injury that occurs after intravascular administration of contrast agents in patients. In recent years, with the widespread development of vascular imaging technology, the risk of CIN has increased. CIN has become the third cause of acquired Acute Kidney Injury (AKI) in hospitals [1,2]. The most commonly used clinically indicator for monitoring changes in renal function is SCr, a marker of Glomcrular Filtration Rate (GFR). However, contrast-induced acute kidney injury is mainly caused by renal tubular injury and rarely occurs in glomeruli. Therefore, the levels of SCr used in the diagnosis of CIN is not an ideal marker[3]. In addition to its anticoagulant function, AntithrombinIII (AT-III) also has strong anti-inflammatory properties, which play an important role in alleviating the development of renal disease [4,5]. The relationship between AT-III levels and the development of CIN has been demonstrated. However, it is unknown whether low AT-III activity increases the susceptibility to CIN. The aim of this study is to investigate the association between initial AT-III levels and the occurrence of CIN, and to evaluate the potential predictive value of AT-III after PCI.
Material and Methods
Study Population
This study selected 300 patients who underwent PCI between September 2018 and June 2019 in the affiliated hospital of Xuzhou medical university. All patients were administered with Iohexol Injection (50ml: 17.5g, Yangtze River Pharmaceutical Group Co., Ltd.), during the operation, and received routine hydration treatment during the perioperative period. The hydration solution was 0.9% normal saline.
Exclusion Criteria
The exclusion criteria for the study were as follows: (1) severe liver and renal dysfunction. (2) Perioperative hemodynamic disturbance and ST-segment elevation myocardial infarction. (3) Patients who had received contrast media within the previous two weeks. (4) Preoperative and hospitalized patients with nephrotoxic drugs. (5) Patients with severe heart failure.
Data Collection
General data form the selected patients: Age; sex; smoking history, past clinical history, including hypertension, diabetes, chronic kidney disease, and the use of the following drugs during hospitalization was recorded, including angiotensin-converting enzyme inhibitors or angiotensin receptor blocker, beta blockers, calcium channel blockers, diuretics, statins, nitrates, and low molecular weight heparin. The contrast volume was recorded during the PCI. Concomitantly, fasting venous blood samples, AT-III activity levels, liver and kidney function, blood glucose, blood lipids and other indicators were measured within 24h after admission, and SCr, cystatin C were measured within 48 to 72 hours after the administration of contrast agent. All the above indexes were measured using Roche Cobas C 701 (Roche Products (Ireland) Limited) automatic biochemical analyzer by the laboratory of the affiliated hospital of Xuzhou medical university. The blood coagulation function samples were determined by Sysmex CS-5100 (Heisenmeikang medical electronics (Shanghai) co., LTD) automatic coagulation analyzer. SCrwas determined by oxidase assay, cystatin C by enhanced immunoturbidimetric assay, and AT-III by hair color substrate assay.
Diagnosis of CIN
According to the latest KDIGO guidelines published by the European Association of Genitour Reproductive Radiology (EUSR) in 2012, the diagnostic criteria for CIN: after exclusion of other influencing factors, within 72 hours after intravascular application of contrast agents, Acute exacerbation of renal failure occurred with a 25% increase in SCr or 0.5 mg/dL (44.2 μmol/L) as the diagnostic criteria[6].
Statistical Analysis
Continuous variables were presented as mean values (Standard Deviation (SD)) or medians with ranges, and the categorical variables were expressed as percentages. The variables were compared using a 2-tailed Student t test for the continuous variables of normal distribution or the Mann-Whitney U test for the continuous variables of nonnormal distribution. A c2 test was used for the categorical variables. Logistic regression was used to evaluate the association between AT-III and CIN by calculating Odds Ratios (ORs) or adjusted ORs with 95% Confidence Intervals (CIs). Pearson correlation for normal variables or Spearman's correlation for skewed variables was used to evaluate the associations between study parameters. The predictive values of SCr, CysC and AT-III were estimated by the areas under the receiver operating characteristic curve. Moreover, the increased discriminative value of AT-III was also estimated using the Net Reclassification Improvement (NRI) and integrated discrimination improvement. All statistical tests were two-sided, and the significance level was set as P<0.05. The statistical analyses were performed using SPSS software, version 19.0 (IBM Corporation, Armonk, NY, USA).
Results
A total of 41 (13.7%) patients developed CIN. The mean age was 63.0(16.2) years. Of all 300 patients, 188 (62.7%) were males and 112 (37.3%) were females. The patients in the CIN group were significantly older (P < 0.05). The number of diabetic patients was also significantly higher in the CIN group (P < 0.05). There were no significant differences in gender, concomitant underlying diseases (such as hypertension, chronic kidney disease), or previous smoking history in both groups. Significant differences were not observed between the CIN and the non-CIN groups regarding the use of angiotensin-converting enzyme inhibitors or angiotensin receptor blocker, beta-blockers, calcium channel blockers, diuretics, statins, nitrates, and low molecular weight heparin. There was no significant difference in the use of contrast media volume between the two groups. The baseline characteristics of the study patients are presented in (Table 1).
The levels of SCr and CysC after PCI were significantly higher in the CIN group. The AT-III levels in the CIN group were lower than non-CIN group (P < 0.001). The levels of AT-III and other laboratory findings are presented in (Table 2).Logistic multivariate regression analysis showed that there was a positive correlation between diabetes and CIN, which was an independent risk factor (OR=3.329, 95% CI: 1.461~7.587, P=0.004); The levels of AT-III were negatively correlated with the incidence of CIN and was a protective factor (OR=0.911, 95% CI: 0.871~0.953, P<0.001).The absolute value of the difference between SCr and CysC after PCI and the baseline levels at admission was calculated, and was expressed as ?SCr and ?CysC, respectively. Pearson correlation analysis showed that AT-III levels was significantly negatively correlated with ?SCr (r = - 0.446, P<0.05)(Table 3).
AT-III levels was negatively correlated with ?CysC (r = - 0.164, P<0.05). The results were shown in (Figure 1).The AUC of AT-III was 0.771 (95% Confidence Interval [CI]: 0.691-0.851, P < 0.001), (Figure 2). The AUC for postoperative SCr was 0.751 (95% CI: 0.674-0.829, P < 0.001) and the AUC of postoperative CysC was 0.626 (95% CI: 0.536-0.716, P < 0.05) for the prediction of CIN, (Figure 3). The best cut-off value of AT-III was 89.5% (Figure 3). According to the best cut-off value of AT-III, patients were divided into high value group (AT-III≥89.5%, n=195) and low value group (AT-III<89.5%, n= 105). The results showed that the sensitivity of AT-III was 80.5% (33/41), the specificity was 72.2% (187/259), and the positive predictive value was 31.4% (33/105), the negative predictive value is 95.9% (187/195).
Discussion
A total of 300 patients were enrolled in the study. According to the KDIGO guidelines for diagnosis of CIN, 41 patients with CIN occurred in this study, with an incidence of approximately 13.7%.By comparing the general data of the included subjects, it can be seen that there is no significant difference between the CIN group and the non-CIN group in terms of gender, contrast media volume, and the history of hypertension or chronic kidney disease. In the previous literature on risk factors for CIN, the Mehran scoring system was developed to assess the incidence of contrast nephropathy with different scores [7]. In Mehran scoring system, previous history of congestive heart failure, diabetes, chronic kidney disease, concomitant hypotension, anemia, excessive contrast media volume, and age > 75 years were all related to the incidence of CIN. In this study, there were no significant inter-group difference in the history of chronic kidney disease and the contrast media volume.
Previous studies have shown that AT-III can reduce the infiltration of F4/80-positive macrophages and reduce the expression of TNF-α, MCP-1 and ICAM-1 mRNA in the kidney, thereby inhibiting cellular oxidative stress and reducing renal cell apoptosis[8,9]. In this study, the levels of AT-III in the non-CIN group were significantly higher than in the CIN group. It is indicated that the high levels of AT-III might play a role of protective effects on the kidney.In order to minimize the incidence of CIN, all patients with intravascular contrast media should be assessed for risk factors. Most risk factors can be derived from clinical history, physical examination and common laboratory results. For high-risk patients, prophylaxis treatment should be considered. In this study, Logistic regression analysis confirmed that the incidence of CIN was closely related to the preoperative AT-III levels. With the increasing of AT-III levels, the incidence of CIN was significantly reduced. The incidence of CIN was significantly higher in patients with diabetes and was an independent risk factor for CIN, which is consistent with previous studies [10].
Because the incidence of CIN was generally low , in order to analysis of AT-III levels for predicting practical clinical value of CIN, we through the fourflod table to calculate the negative predictive value(95.9%), reached a high levels, and it shows that when the AT-III levels above the 89.5%, which can effectively eliminate the incidence of CIN, in the case of AT-III levels under 89.5%, although the positive predictive value of only 31.4%, however, it is still necessary to pay attention to the monitoring of postoperative SCr in this group of patients.So far, the exact pathogenesis of CIN has not been fully studied. At present, it is believed that direct cytotoxicity, renal hypoperfusion, inflammatory response, oxidative stress and endothelial dysfunction of the pathogenesis of CIN [11-13]. The contrast media can stimulate the body to produce a large number of neutrophils by mediating the inflammatory reaction [14-16].
On the one hand, neutrophils can release active oxygen, which leads to increased renin secretion, causing renal vascular lesions, and on the other hand, produces a large number of inflammatory mediators in the renal vascular endothelial cell surface adhesion, lead to renal vascular endothelial dysfunction, cause kidney damage[17]. AT-III is a major inhibitor of coagulation protease, mainly synthesized by liver, vascular endothelial cells and macrophages. In addition to anticoagulant function, AT-III also has strong anti-inflammatory properties and cytoprotective function. Its anti-inflammatory function plays an important role in alleviating the development of kidney disease[18]. Previous studies have shown that exogenous AT-III has protective effects on liver, lung, kidney and myocardial damage, while the deficiency of endogenous AT-III can aggravate renal ischemia-reperfusion damage [19-21]. Low levels of AT-III are closely linked to the prognosis of related diseases and are considered to be an important prognostic indicator[22].
At present, the pathophysiological mechanism of AT-III levels and CIN is still unclear. One possible mechanism is that the decrease of AT-III levels can lead to a decrease in renal blood flow, and AT-III can stimulate endothelial cell release. The same studies believe that Prostaglandin I2 (PGI2) has vasodilation, can regulate renal cortical blood flow, increase renal blood flow, improve renal hypoperfusion, and prevent renal damage [23,24]. In addition, a decrease in AT-III levels can result in a hypercoagulable state of the blood, promote thrombosis, and can lead to impaired renal function and glomerular sclerosis [25]. Another possible mechanism is that when AT-III levels is reduced, the inflammatory response is enhanced. Previous study had shown that AT-III has a strong anti-inflammatory effect, and its anti-inflammatory effect is mainly to inhibit leukocyte aggregation and activation of the nuclear factor-κB pathway, thereby inhibiting the production of cytokines [26]. Therefore, when AT-III levels is at a low level, the inflammatory response and renal endothelial cell hypoxia are significantly enhanced after administration of the contrast agent, resulting in oxygen free radicals, elastase,myeloperoxidation.
A variety of inflammatory mediators such as enzymes, a large number of inflammatory mediators adhere to the surface of renal vascular endothelial cells, leading to renal vascular endothelial dysfunction, thereby aggravating renal damage. However, the above possible mechanism is speculative, and the detailed molecular mechanism of the decrease in AT-III levels leading to the occurrence of CIN should be further studied.CIN is a practical clinical problem that may not be fully understood due to the limitations of existing biomarkers [27]. Although CIN is a transient renal impairment in patients, it cannot be ruled out that some patients with CIN develop significant persistent renal impairment [28-30]. Current interventions focus on hydration therapy, avoiding the use of nephrotoxic drugs and optimal selection of contrast media, which have limited effectiveness in preventing CIN.
A recent meta-analysis showed hydration with sodium bicarbonate, the use of supplements such as N-acetylcysteine and vitamin C, and the administration of statins and adenosine antagonists are beneficial in the prevention of CIN after PCI[31]. Our research still has some limitations. First, the number of patients included is relatively small, especially in patients with low AT-III levels. Therefore, a larger sample size study is needed to reveal the clinical significance of AT-III levels in predicting CIN. Second, our study failed to follow-up patients with CIN. The association between AT-III levels and short-term or long-term prognosis in patients with CIN could not be further evaluated. Further follow-up studies should reveal the predictive value of AT-III levels in the prognosis of patients with CIN.
Authors’ Note
All authors contributed to (1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and (3) final approval of the version to be published.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
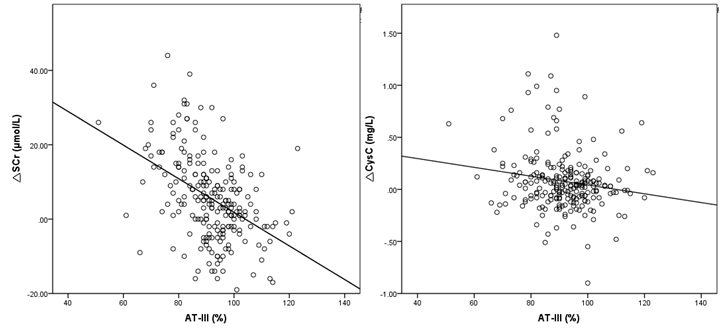
Figure 1:AT-III levels and ?SCr, ?CysC correlation analysis scatter diagram.
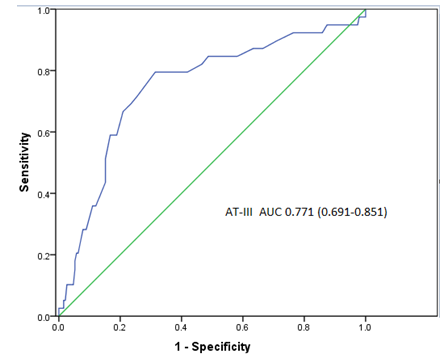
Figure 2: Receiver Operating Characteristic (ROC) curve analysis for AT-III levels in predicting Contrast-Induced Nephropathy (CIN) development.

Figure 3: Receiver Operating Characteristic (ROC) curve analysis for SCr and CysC after surgery in predicting Contrast-Induced Nephropathy (CIN) development.
|
Variable |
CIN group (n=41) |
Non-CIN group (n=259) |
P |
|
Age, years |
69.3±11.0 |
62.0±10.1 |
0.032 |
|
Male, n (%) |
27 (65.9) |
161 (62.2) |
0.645 |
|
Smoking history, n (%) |
18 (43.9) |
75 (29.0) |
0.138 |
|
Hypertension, n (%) |
8 (19.5) |
113 (43.6) |
0.057 |
|
Diabetes mellitus, n (%) |
27 (65.9) |
85 (32.8) |
<0.001 |
|
Chronic kidney disease, n (%) |
2 (4.9) |
12 (4.6) |
0.974 |
|
Medication |
|
|
|
|
ACE-I or ARB, n (%) |
21 (51.2) |
124 (47.9) |
0.958 |
|
β-blocker, n (%) |
18 (43.9) |
105 (40.5) |
0.982 |
|
CCB, n (%) |
12 (29.3) |
65 (25.1) |
0.647 |
|
Diuretic, n (%) |
14 (34.1) |
69 (26.6) |
0.494 |
|
Statins, n (%) |
36 (87.8) |
198 (76.4) |
0.725 |
|
Nitrates, n (%) |
22 (53.7) |
136 (52.5) |
0.952 |
|
LMWH, n (%) |
13 (31.7) |
72 (27.8) |
0.69 |
|
Contrast media volume (mL) |
104.7±46.5 |
95.6±54.3 |
0.149 |
|
Abbreviations: ACE-I: Angiotensin-Converting Enzyme Inhibitors; ARB: Angiotensin Receptor Blocker; CCB: Calcium Channel Blockers; LMWH: Low-Molecular-Weight Heparin; CIN: Contrast- Induced Nephropathy. |
|||
Table 1: Baseline Characteristics of the Study Population (x?±s).
|
Variable |
CIN group (n=41) |
Non-CIN group (n=259) |
P |
|
TG (mmol/L) |
1.28±0.91 |
1.60±0.90 |
0.051 |
|
TC (mmol/L) |
4.39±1.17 |
4.50±1.25 |
0.597 |
|
LDL-C (mmol/L) |
2.63±0.98 |
2.72±1.09 |
0.666 |
|
HDL-C (mmol/L) |
1.20±0.27 |
1.17±0.36 |
0.594 |
|
FBG (mmol/L) |
6.77±2.88 |
6.82±2.65 |
0.916 |
|
AT-III (%) |
83.6±11.4 |
93.3±10.2 |
<0.001 |
|
FIB (g/L) |
3.11±0.85 |
3.10±0.90 |
0.968 |
|
HbA1c (%) |
7.25±1.95 |
6.88±1.44 |
0.238 |
|
Renal function before PCI |
|
|
|
|
BUN (mmol/L) |
5.80±1.72 |
5.73±1.93 |
0.822 |
|
SCr (μmol/L) |
66.05±15.27 |
69.33±17.35 |
0.256 |
|
UA (μmol/L) |
298.81±80.98 |
312.68±93.29 |
0.393 |
|
CysC (mg/L) |
0.78±0.17 |
0.80±0.17 |
0.489 |
|
eGFR (mL/min) |
106.3±27.6 |
100.4±24.3 |
0.162 |
|
Renal function within 48-72h after PCI |
|
|
|
|
BUN (mmol/L) |
5.74±2.68 |
4.51±1.67 |
<0.001 |
|
SCr (μmol/L) |
88.66±20.47 |
71.10±19.38 |
<0.001 |
|
UA (μmol/L) |
341.86±112.73 |
296.96±96.53 |
0.028 |
|
CysC (mg/L) |
0.90±0.23 |
0.82±0.20 |
0.023 |
|
eGFR (mL/min) |
75.5±20.1 |
97.8±25.7 |
<0.001 |
|
Abbreviations: CIN:Contrast-Induced Nephropathy; TG:Triglyceride; TC:Cholesterol; LDL-C:Low-Density Lipoprotein; HDL-C:High-Density Lipoprotein; FBG:Fasting Blood Glucose; AT-III:AntithrombinIII; FIB:Fibrinogen; HbA1c:Glycosylated Hemoglobin; BUN:Blood Urea Nitrogen; SCr: Serum Creatinine; UA: Uric Acid; CysC: Cystatin C; eGFR: Estimated Glomerular Filtration Rate |
|||
Table 2: The Laboratory Findings of Study Population (x?±s).
|
|
Univariate |
Multivariate |
||
|
Variables |
OR (95% CI) |
P |
OR (95% CI) |
P |
|
Age, years |
1.015(1.007-1.018) |
0.032 |
1.004(0.956-1.012) |
0.078 |
|
Hypertension |
2.181(0.963-4.936) |
0.057 |
1.605(0.618-4.168) |
0.331 |
|
Diabetes |
3.517(1.751-7.065) |
<0.001 |
3.329(1.461-7.587) |
0.004 |
|
TG(mmol/L) |
0.558(0.311-1.004) |
0.051 |
0.792(0.442-1.419) |
0.433 |
|
AT-III(%) |
0.914(0.881-0.947) |
<0.001 |
0.911(0.871-0.953) |
<0.001 |
Table 3: Univariate and Multivariate Logistic Regression Analysis for CIN.
Citation: Lu J, Sun Y, Li Y, Zheng D, Zhang Q, et al. (2020) The Prediction Value of Antithrombin III on the of Contrast-Induced Nephropathy in Coronary Artery Disease Patients Undergoing Percutaneous Coronary Intervention. J Urol Ren Probl: JURP-100011.