Journal of Surgery and Insights
(ISSN 2652-4643)
Case Report
Fat Graft Associated with Negative Pressure Wound Therapy: Case Report
Souza, GMCI*, Amorim CCBII, Faria KCMII, Vallejo CEAII, da Costa SMIII, Sternick MBIVSobral CSV and Ferreira LMVI
IDepartment of Surgery, Universidade Federal de São Paulo (UNIFESP), Brazil
IIDivision of Plastic Surgery, Hospital Felicio Rocho, Belo Horizonte-MG, Brazil
IIIDivision of Plastic Surgery, Hospital Felicio Rocho, Belo Horizonte-BH, Brazil
IVDivision of Orthopaedic Surgery, Hospital Felício Rocho, Belo Horizonte-BH, Brazil
VDivision of Plastic Surgery, Hospital dos Defeitos da Face-Cruz Vermelha Brasileira, São Paulo-SP, Brazil
VIDivision of Plastic Surgery, UNIFESP, Researcher 1A CNPq, Director Medicine III-CAPES, São Paulo-SP, Brazil
*Corresponding author: Gustavo Moreira Costa de Souza,Department of Surgery, Universidade Federal de São Paulo (UNIFESP), Brazil
Citation:Souza GMC, Amorim CCB, Faria KCM, Vallejo CEA, da Costa SM, et al. (2020) Fat Graft Associated with Negative Pressure Wound Therapy: Case Report. J Surg Insights: JSI-100024
Received date: 16 July, 2020; Accepted date: 03 August, 2020; Published date: 10 August, 2020
Abstract
Introduction:Complex wounds represent a fundamental and prevalent theme in plastic surgery. In the 21stcentury new treatment options have emerged, such as the negative pressure wound treatment (NPWT), the fat graft (FG) and the biological matrix.
Objective: To describe a case report of FG application with NPWT in the treatment of complex wound on the distal third of the lower limb with bone exposure.
Case report: A 59-year-old patient with chronic left tibial osteomyelitis since childhood underwent extensive debridement of the distal tibial diaphysis and placement of bioactive glass (40% of bone thickness per 10 cm extension). There was distal necrosis in the fasciocutaneous flap used as the primary bone coverage. After flap debridement, the case was resolved with FG, directly on exposed bone and biomaterial, associated with NPWT. Three weeks after the first FG session, 100% granulation was achieved over bone and bone marrow. The closure was completed with thin laminated skin graft over the wound surface.
Discussion: The association of fat grafting and NPWT for inferior limb reconstruction was unpublished in clinical use. Except for only one experimental study described by Kao et al. (2015) that addressed the theme. In this clinical case, the result obtained regarding the granulation velocity drew attention, which avoided the use of more complex flaps such as the microsurgical.Accelerated granulation tissue formation was observed, filling an extensive and deep bone defect, even with infected bone and exposed biomaterial. Low morbidity and no complications were observed with the use of FG associated with NPWT. Also, when the grafted fat was aconditionated with the NPWT it seemed to behave as a true autologous biological matrix with large amount of cells. To date,studies on fat grafting have focused on the cellular aspect (adipocytes and mesenchymal cells), as well as growth factors and fat differentiation in different tissues. The property of adipose tissue as a biological matrix appeared to have been intensified by the application of NPWT and may represent a new study line in plastic surgery.
Keywords:Wound healing, Fat transfer; Autologous Transplantation; Negative Pressure Wound Therapy
Introduction
Wounds have been a fundamental and prevalent theme in plastic surgery since the beginning of its history [1]. For its treatment, surgical techniques were created, in a progressive degree of complexity. From the simple suture, through the skin graft, from there to the local flap, rising to the distant flap, until it reaches the transfer of flaps by microsurgical technique[2]. In complex wounds with bone exposure or other noble tissues such as the dura mater, vessels, nerves and tendons, microsurgical flaps often represent the only alternative.These can prevent amputation of a limb or even save a life.Important to note that wound care options have suffered continuous evolution. In the 21stcentury, for example, among the top ten technological innovations in plastic surgery, there have been three advances within the theme of cicatrization: negative pressure wound therapy (NPWT), fat transfer and biological matrices.
NPWT (-125mmHg) is known to stimulate blood circulation in the wound bed, reduces dead space and edema, eliminates exudate, accelerates granulation, and helps to fight infection. It is indicated for infected wounds such as mediastinitis and fasciitis. It should not be applied to a wound with tissue necrosis, malignant disease and unexplored osteomyelitis.
Fat grafting hasgained notoriety and thelipotransfer technique (FG) boosted in current medical practice. It is already known of the angiogenic, immunomodulatory, anti-inflammatory, analgesic, regenerative and filling effects that can be obtained with adipose tissue. FG changed the routine of wound treatment in the distal third of the lower limbs with open fracture. Higher elasticity and malleability of scarred areas were observed, with functional and aesthetic benefit in patients after treatment of cicatricial contractures[3].
Cellular support and stimulation from the extracellular matrix is already known in cicatrization studies. In FG, this quality was also noticed, because adipose tissue not only carries adipocytes and multipotent mesenchymal cells. This interstitial microanatomic condition with intercellular metabolic activity through a reticulum that supports and connects cells, has been termed by as a “microconnected fat graft”.
The biological matrices were inspired by the structural property of extracellular matrix of natural tissues such as the dermis. They act as a support for granulation tissue formation to cover exposed noble structures and seek to improve the thickness of a wound bed. It is useful in large substance losses or in large burns with scarring contractures and high risk of relapse[4].
In complex wounds, such as distal lower limb trauma with severe substance loss, infection, and bone exposure, these three techniques can be applied. Their properties are synergistic in the healing process, so that if used together they can have their effects optimized. Negative pressure therapy and acellular dermal matrix have already been associated with faster integration of the latter. Negative pressure and autologous skin graft have also been associated. However, no clinical studies exists in the literature on the combination of negative pressure and autologous fat graft for inferior limb reconstruction. Only one experimental study on guinea pigs skull studied this association, with very favorable results[5].
The aim of this study is to describe a case report with the association of concomitant autologous fat grafting and negative pressure wound therapy.
Case Report
A 59-year-old male patient with a history of chronic left leg injury due to chronic tibial osteomyelitis since he was eight years old, after orthopedic surgery on site. No other complaints. Normotensive and without diabetes mellitus. On physical examination, the patient presented with a 6 cm long vegetating ulcerated lesion on the distal anterior aspect of the left leg. MRI showed signs of inflammatory / infectious process (osteomyelitis) in activity in the left tibia.
On 4/18/2019 he underwent extensive debridement of the left tibia (40% of bone thickness per 10 cm extension), together with the associated dystrophic skin. For the treatment of chronic osteomyelitis, we opted for the inclusion of bioactive glass (BAG-S53P4) in the bone marrow. For coverage of the exposed tibia, rotation of the extended fasciocutaneous flap of the fascia of the left medial gastrocnemius muscle, with interest in the posteromedial aspect of the lower limb, was chosen[6].
Antibiotic therapy with ciprofloxacin and clindamycin was started. Pathological examination of the material removed from the left tibia revealed findings compatible with chronic osteomyelitis extending to the skin and soft tissues (connective-vascular neoformation and necrosis). No signs of malignancy, granulomas or parasitic agents[7].
On 04/25/2019 partial flap ischemia was confirmed fasciocutaneus on the distal anterior aspect of the left leg(Figure 1A-H).
On 05/03/2019, after a good delimitation of necrosis and the appearance of purulent discharge from the surgical wound, the patient underwent an extensive debridement of necrotic tissue, with partial preservation of BAG-S53P4, by recommendation of the orthopedic team.
An extensive complex infected wound with exposure of biomaterial, bone marrow and tibial cortex was found. The culture revealed Morganella morganii and Enterococccus faecalis. (Figure 1B) Specific venous antibiotic therapy (Ampicillin and Cefepime) was adjusted, with sensitivity confirmed by the antibiogram, according to the chronic osteomyelitis treatment protocol (ninety days). Without the option of oral antibiotic therapy[8].
On May 7, 2019, after removal of residual tissue necrosis, it was decided to temporarily apply TFPN until the definition of a microsurgical flap or a cross-leg fasciocutaneus flap. The permanence of the vacuum dressing with the same reservoirwas left in place for 7 days.
On 05/13/2019, the wound was still without any perspective of a microsurgical flap and remained with exposure of biomaterial, bone marrow and tibial cortical bone (Figure 1C). We opted for grafting aspirated and decanted fat (30cc) over the wound bed. A large volume of adipose tissue sufficient to fill in
the entire defect (Figure 1D). Associated with fat grafting, vaselinated gauze coverage(Adaptic ®) and NPWT were used for another week. The fat donor area was the medial face of the right thigh. An aspiration cannula (4mm x 25cm) and a 60cc syringe for fat aspiration (seringo-vac) were used.
On May 21st, 2019, after seven days, during dressing removal, there was no fat in the wound and a large increase in granulation (Figure 1E). The right thigh donor area evolved appropriately, with progressive regression of local ecchymosis. With the significant improvement of the wound and the low morbidity observed, we opted for a second fat grafting session (30 cc), associated with the application of NPWT. The fat donor area was the medial face of the left thigh, in the same way as the first collection.
On 05/30/2019, progressive granulation was observed, except for the central area, which persisted with purulent discharge and granules of infected biomaterial. It was the only site where a small loss of grafted fat was noted (Figure 1F). With the exuberance of bleeding and granulation tissue already obtained, in addition to filling the defect, it was decided to continue the NPWT for another week.
On 06/06/2019, the total granulation of the wound was observed, including over the bone marrow area, without bone or BAG-S53P4 biomaterial exposure (Figure 1G). Laminated thin skin grafting was performed to close the woundsurface. Samples of the newly formed granulation tissue were collected for pathological examination, which revealed an inflammatory/reparative process, compatible with the transformation of grafted fat into granulation tissue[9].
On 6/13/2019, 30 days after the start of FG associated with NPWT, 100% wound epithelialization was observed (Figure 1H). The left thigh skin donor area followed with progressive epithelialization without complications. In all negative pressure dressings performed (1 dressing/week) there was no need for reservoir replacement. Wound with little exudate, even with fat grafting applied (30 cc per session). The patient remained feverless throughout the treatment.
On June 14, 1919, the hospital was scheduled for discharge by the plastic surgery and outpatient control team. Radiographic control was performed by the orthopedics team, with the good amount of BAG-S53P4 remaining (Figure 2)Postoperative control of 19/07/2019.
On June 14, 2019, the patient received hospital discharge. Radiographic control was performed by the orthopedics team, with a good amount of BAG-S53P4 remaining (Figure 2).
One year after the operation, the lesion is completely closed, with no loss of graft areas, no signs of infection, considering the result to be satisfactory (Figure 3).
Discussion
In the treatment of complex lower limb distal third wounds, fasciocutaneous flaps are the only option for reconstruction with local tissues. In this case, despite 50 years of successive wounds and unstable healing in the distal portion of the left tibia due to chronic osteomyelitis, an extended posterior medial fasciocutaneous flap of the proximal pedicle was performed, with extension of the left medial gastrocnemius muscle fascia, which unfortunately had na unfavorable evolution with anterior partial necrosis. In the current literature, fasciocutaneus flaps of the distal reverse pedicle have gained more importance and clinical application, with a high success rates[10].
However, both may evolve with total or partial losses. Once the local flap fails, the microsurgical flap has become the most appropriate option in any plastic surgery service with available microsurgery team. The indication of fat grafting, in conventional technique, for this complex wound, with the topic apposition of free aspirated fat over the wound, was a great exception, although there are already reports in the literature of the application of fat grafting in wounds. In addition to debridement of infected bone tissue in the left tibia, the orthopedic team used BAG-S53P4 bioactive glass, which demonstrated effectiveness and low complication rates in the treatment of chronic osteomyelitis[11].
This biomaterial was developed as a synthetic bone substitute with bactericidal properties. It allows the dissolution of ions, which alkalinize the environment where it is used, fighting panctonic bacteria in biofilm. In addition it was revealed osteoconductive material, serving as a framework for bone neoformation. It also promotes the release of angiogenic growth factors, important for angiogenesis. However, as with all biocompatible synthetic material, it is essential for its integration to be coveredwith a flap or vascularized tissue. The indication of fat grafting for its coverage was one more exception.
Although these are known procedures and applied more recently, with the purpose of tissue regeneration, the association of concomitant fat grafting with NPWT has been unprecedented in the medical literature in humans for lower limb reconstruction. Kao et al.[12]defended this treatment in an experimental study carried out on guinea pig’s exposed skulls. The grafted fat was removed from the guinea pig's inguinal cushion.A group with one session and one group with two sessions of fat grafting was described, with a 4-day interval for dressing change. They observed better granulation in the second group.They observed 100% fat loss and no granulation in the minced fat graft group, without association with NPWT[12].
A clean wound without residual necrosis is critical to any type of graft. TFPN wound conditioning for a week prior to fat grafting increased local blood circulation and helped clear wound fibrin remnants and exudates, improved conditions for a graft. At that time, the wound also had some granulation on its margins.
In this clinical case, it was decided to standardize the dressing change once a week. This time represented the maximum durability recommended for a negative pressure dressing without change, as well as ensuring the longest possible immobilization interval for the fat graft, optimizing its healing and the cost of treatment. The little exudate observed (less than 300 ml in seven days) also facilitated the maintenance of the dressing.
Experimental studies have taught that the process of fat healing involves the death of the vast majority of mature adipocytes grafted and their replacement by fibroblasts and granulation tissue. In this clinical report, however, the speed of this granulation process, the low volume of accumulated exudate, the absence of steatonecrosis detected, and a macroscopic loss of small amounts of fat grafted oberved during the third NPWT exchange (after the second graft). The site with loss of EG, coincided with medullary bone area with bioglass granules (Figure 1F). The large difference in granulation tissue from the first postoperative week from the first FG was notorious(Figure 1C and 1E).
The result of the speciments examination of the collected granulation tissue, revealed its origin from adipose tissue, which may suggest the presence of adipocytes or their metaplasia, rather than simple apoptosis or death, as other authors have argued(Figure 4).
The method applied in this work was the FG directly over the wound, different from the structured FG of which advocates the construction of several narrow tunnels and small volumes (1cc per tunnel) grafted under the skin. Due to the depth of the defect and the large amount of graft (30 cc per session), the fat remained as a supernatant fluid over the left tibia (Figure 1D). In this situation, all the fat would have been lost due to ischemia and bacterial aggression, not to mention the need fordaily dressing changes, with additional losses due to manipulation.
NPWT application reduced dead space, eliminated residual serosity of grafted tissue, stabilized and aconditionated adipocytes near the wound bed. To ensure its survival , it is known that the adipocyte must remain 2mm (up to a maximum of 4mm) from the nearest vascular bed. The effect of fat graft aconditioning under NPWT, may have been compatible with the NPWT's ability to increase local blood flow. Sufficient nutrition and stability were observed for neoangiogenesis with exuberant granunulation of the grafted tissue (Figure 1G). In addition, bacterial action was counteracted with continuous exudate drainage and specific antibiotic therapy.
In this clinical case, the grafted fat was collected by liposuction and decanted without any additional preparation. There is no evidence of superiority between current fat processing methods. However, when considering the concept of the “cell niche” that argued for the importance of interstitial microanatomy of aspirated adipocytes, different methods of fat collection, preparation or separation may make a significant difference. In the clinical case in question, the grafted fat soon became good quality bone coverage tissue with easy bleeding, a behavior very similar of a “microconnected biological matrix”.This new reality may have been possible to achieve by the action of NPWT[13,14].
The aconditioning of large amounts of fat, also determined the overlap of its extracelular interstitial portion. In macroscopic view, the thickness of this tissue appears to have corresponded to that of a true biological matrix, with its increased capacity as an extracellular framework for structural support and cell migration. In addition, different from the caractheristics of acellular heterologous matrices, an environment of autologous biological matrix with high cellular metabolic activity was achieved, with viable adipocytes and mesenchymal cells connected in their overlapping extracellular micro-matrices.
The leg remains epithelized for twelve months. However, due to the history of osteomyelitis, wound infection and permanence of the biomaterial, long-term follow-up is expected with the termination of specific antibiotic therapy. Orthopedics team orientation was to maintain only local care and use of Sarmiento orthosis during support, due to the great tibial bone loss. The whole treatment was based on new technologies for the treatment of wounds, without the use of microsurgical flap technique, which in the past would probably be the only alternative for the treatment of this leg with chronic osteomyelitis.
Adipocytes, mesenchymal cells and extracellular interstitial matrix proved to be an indivisible functional unit. There is little research on the matrix property of aspirated fat, even because of the need for its aconditioning in bigger amounts, in order to achieve sufficient thickness, to function as a biological matrix for bone coverage, was only made possible due to the effects of NPWT. This new approach has great potential for further study and treatment of even more complex and irregular injuries than those indicated for acellular heterologous dermal matrices.
Acknowledgments
We thank the support of the pathology laboratory of Hospital Felício Rocho for the histological studies performed.
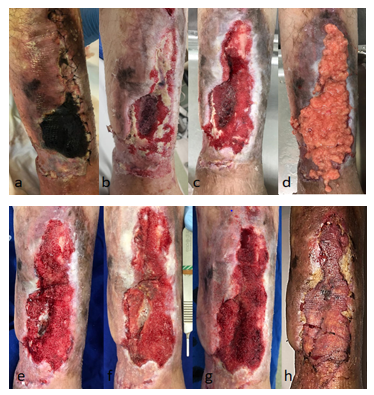
Figure 1: A-H Evolution of the left leg (distal portion) during treatment. Arrow: bioglass with bone marrow.
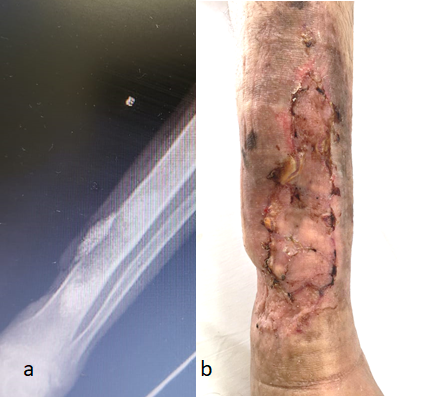
Figure 2: Postoperative control of 07/19/2019.A: radiological aspect. B: macroscopic aspect Arrow: Bioglass on the area of bone debridement.
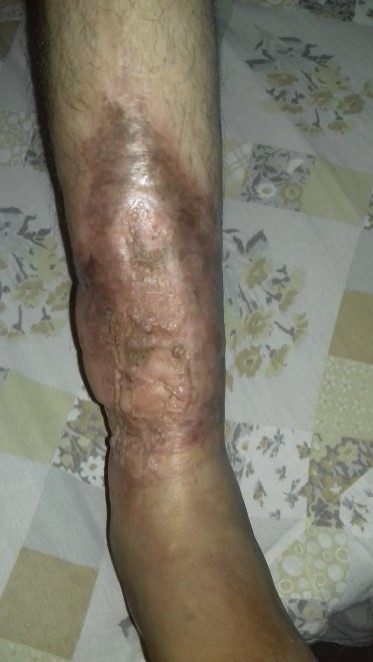
Figure 3: Late postoperative control (1 year), macroscopic appearance. Photo sent by the patient.
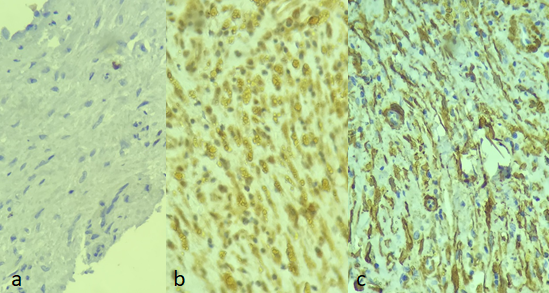
Figure 4: Immunohistochemistry of fat-formed tissue. A: KI 67 VEGF Actin. B: VEGF: Endothelial Vascular Growth Factor. C: Actin: marker for smooth muscle and myofibroblasts
Citation:Souza GMC, Amorim CCB, Faria KCM, Vallejo CEA, da Costa SM, et al. (2020) Fat Graft Associated with Negative Pressure Wound Therapy: Case Report. J Surg Insights: JSI-100024