Argyria in a Pediatric Patient Ingesting Silver Nitrate
Christine Do1*, Taylor E Nickerson2, Alejandro Llanos-Chea2, Harland S Winter2
1*Department of Pharmacy, Massachusetts General Hospital, USA
2Department of Pediatric Medicine, Massachusetts General Hospital, Harvard Medical School, USA
*Corresponding author: Christine Do, Department of Pharmacy, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, USA, Tel: +16177262502.
Citation: Do C, Nickerson TE, Llanos-Chea A, Winter H (2019) Argyria in a Pediatric Patient Ingesting Silver Nitrate. Ann Pediatr Child Care: APCC-100001.
Received Date: 26 November, 2018; Accepted Date: 11 January, 2019; Published Date: 21 January, 2019
1.Abstract
Historically used for its putative antimicrobial properties, chronic ingestion of silver can result in argyria, characterized by discoloration of skin and mucous membranes. We report the case of a pediatric patient with generalized argyria resulting from silver nitrate consumption as alternative treatment for Crohn’s disease. Two years after ingestion stopped, serum silver levels remain elevated.
2. Keywords: Blue; Child; Ingestion
3. Abbreviations
CD : Crohn’s Disease
FDA : Food and Drug Administration.
4. Introduction
Oral silver preparations have historically been used for their antimicrobial properties; however, this was not without consequence. Avicenna (980-1037 AD) reported patients developing a bluish discoloration of the eyes associated with the ingestion of silver formulations. In 1840, the term argyria was introduced to describe this specific discoloration.1 We report the case of a 10-year-old boy with Crohn’s Disease (CD) who developed generalized argyria following chronic silver ingestion.
5. Case Presentation
A 10-year-old Caucasian boy presented with perianal fistula and poor weight gain and was diagnosed with ileocolonic CD.He was treated with oral azathioprine which was stopped due to leukopenia. He had no immunizations after one year of age and his family sought alternative medical and nutritional therapies. He was a chronically ill-appearing and pale boy, with a blue skin discoloration of his face. In addition to metronidazole, naltrexone, and homeopathic remedies, his parents had been giving him silver nitrate solution (600 parts-per-million, marketed for topical use) at a dose of 2.5 mL by mouth three times a day for the past five years. The silver nitrate was purchased from an online distributor who specifically claimed that their formulation would not cause blue discoloration of the skin.
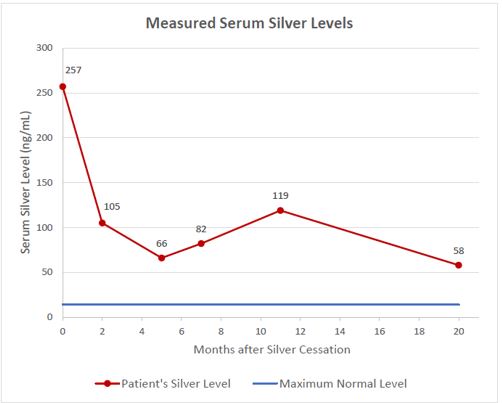
The serum silver level was 257 ng/mL or 2382.5 nmol/L (normal range 0-14 ng/mL) and a diagnosis of generalized argyria was made. Silver nitrate was discontinued and two months later a repeat serum silver level was 105 ng/mL (973.4 nmol/L), but he continued to have a perioral bluish discoloration and a blue tinge of his face. One year later, the argyria had mostly resolved, but the silver level continued to be elevated. The serum silver level 20 months after stopping the silver nitrate remained elevated at 58 ng/mL (537.68 nmol/L) (Figure 1).
6. Review of Literature and Discussion
The historical use of silver has been documented in medical literature. Prior to the discovery of bacteria, silver vessels were used to carry water for Persian kings in the belief that the water would be kept fresher. In the 1880’s, the obstetrician Karl S. Crede proposed the use of silver nitrate 1% eye drops in newborns to prevent gonorrheal ophthalmia. The therapy proved to be effective and the incidence decreased from 7.8% to 0.13% [5]. Silver nitrate eye drops were subsequently advocated as a treatment choice by the United States Centers for Disease Control and the American Academy of Pediatrics and mandated by state law throughout most of the United States up until the 1970’s to prevent gonorrheal ophthalmia in neonates [6]. Despite its antimicrobial properties when applied topically, today silver is considered by the Food and Drug Administration (FDA) as a “nonessential mineral that has no known physiological functions or benefits when taken orally” [7].
The use of silver preparations eventually resulted in a unique adverse effect. Argyria originates from the Latin word for silver, argentum, and is characterized by bluish-gray to slate-gray discoloration of tissues caused by deposits of silver granules in the skin and mucous membranes. Argyria presents in two forms: generalized or localized. Generalized argyria may result from silver absorption through mucosal membranes, injection, inhalation due to occupational exposure, or ingestion; whereas, localized argyria stems mainly from external contact through the skin and may also result in discoloration of the fingernails, conjunctival membranes, and/or the oral mucosa [1].
While argyria is identified by particulate silver accumulation in skin and mucous membranes, other tissues may also be affected. Autopsies of adult patients demonstrated granular deposits of silver within the epidermis, sweat ducts, pituitary gland, myocardium, liver, spleen, adrenals, prostate, thyroid, kidneys, and gray matter of the cerebrum [8,9]. A biopsy of the colonic mucosa in a 73-year-old male presenting with argyria and a 5-year history of colloidal silver ingestion revealed silver granules in the lamina propria and basement membrane of the duodenal epithelium [10]. Most reports describing argyria due to chronic silver consumption are of adults [1,9-24]. Of the few reported children with argyria was an 11-year-old boy with cystic fibrosis who was given colloidal silver to treat his lung disease. His serum silver concentration at the time of argyria diagnosis was 32 ng/mL (296.7 nmol/L), with a follow-up level of 2.1 ng/mL (19.5 nmol/L) nine months after discontinuation. The skin discoloration had resolved [25]. It seems that argyria can persist despite even lower blood concentrations of silver: an adult male with discoloration of the skin and nailbeds had a serum level of just 8.3 ng/mL (76.9 nmol/L) [23]. The trend of silver excretion from the body is not well known. Most reports describe patients with argyria with a single serum silver level, but there are two reports of patients with multiple levels [24,25].
The lack of evidence regarding oral use of silver products resulted in warnings for disease treatment. In 1975, the United States Pharmacopeia and the National Formulary removed colloidal silver products from their guidebooks, and Goodman and Gillman: The Pharmacological Basis of Therapeutics 1980 edition stated that the “indiscriminate use of colloidal silver solutions…probably does more harm than good” [26]. In 1999, the FDA issued a “final rule establishing that all over-the-counter drug products containing colloidal silver ingredients or silver salts for internal or external use are not recognized as safe and effective and are misbranded” [27]. A decade later, the FDA released a consumer advisory warning describing the risks of argyria associated with the use of silver-containing products, adding that silver supplements may interfere with the proper absorption of certain drugs [7]. Currently, the only FDA-approved silver product is silver sulfadiazine 1% topical cream, a prescription drug for the prevention and treatment of wound infections in second and third degree burns [28].
Despite these public alerts, the Internet serves as a means for proprietors to make exaggerated claims regarding the medicinal benefits of silver preparations without revealing potential side effects of their products [2]. The FDA and Federal Trade Commission sent warnings to companies who exert false claims that silver “treats”, “cures”, or “prevents” disease, yet these businesses have found ways to avoid responsibility by marketing the products as “dietary supplements”, a category that allows the product to be legally recognized as intended for oral ingestion by The Dietary Supplement Health and Education Act [2,27]. Silver products for oral administration continue to be sold on the Internet, claiming to treat over 600 different diseases and disease-causing pathogens [26,27]. With the recent rise in silver use, the recurrence of argyria may yet again become a familiar clinical diagnosis.
Silver nitrate was given orally with the unsubstantiated belief that it was preventing and alleviating flares of CD. Elevated serum silver levels decreased upon discontinuation, yet after nearly two years have not returned to the normal range. Despite the distributor’s claims that their specific formulation “won’t turn you blue”[29], the patient’s generalized argyria has persisted.
7. Conclusion
We report generalized argyria resulting from the chronic ingestion of silver nitrate solution administered as an alternative treatment for inflammatory bowel disease. Information on the Internet may not be evidence-based leading to the use of therapies that are unproven to be effective. Increased awareness about parental practices regarding unregulated products and more stringent regulations about Internet content are needed.
|
Potential Sources of Exposure to Silver[1-4] |
|
Non-FDA approved dietary supplements and nasal sprays containing silver Occupational exposure: silver mining, silver refining, silver reclamation, jewelry production, photographs Jewelry Topical silver sulfadiazine Dental amalgams Medical instruments Silverware utensil |
Citation: Do C, Nickerson TE, Llanos-Chea A, Winter H (2019) Argyria in a Pediatric Patient Ingesting Silver Nitrate. Ann Pediatr Child Care: APCC-100001.