Current Updates in Dermatological Problems
A New Synthetic Endocannabinoid as Anti-Inflammaging Cosmetic Active: an In Vitro Study on a Reconstructed Skin Model
Semenzato A1*, Meloni M2, Caviola E2, Galizia G3 and Baratto G4
1Department of Pharmaceutical and Pharmacological Sciences, University of Padova, Italy
2Vitroscreen srl, Italy
3UniR&D srl, Italy
4UNIFARCO S.p.A, Italy
*Corresponding author: Semenzato A, Department of Pharmaceutical and Pharmacological Sciences, University of Padova, Via Francesco Marzolo 5, 35131 Padova, Italy, Tel: +390498275356.
Citation: Semenzato A, Meloni M, Caviola E, Galizia G and Baratto G (2018) A New Synthetic Endocannabinoid as Anti-Inflammaging Cosmetic Active: an In Vitro Study on a Reconstructed Skin Model. Curr Updates Dermatol Probl: CUDP-100003.
Received date: 09 December, 2018; Accepted Date: 28 December, 2018; Published Date: 07 January, 2019
1. Abstract
Endocannabinoids have been recently appointed as interesting cosmetic actives in regulating inflammaging, a state of chronic low-grade inflammation, known for being involved in many senescence’s manifestations, included skin aging. The aim of this study was to assess the anti-inflammaging activity of a new synthetic endocannabinoid, Isopalmide®, on a reconstructed skin model, on which inflammaging has been reproduced through UVA radiation and light mechanical stress. We tested Isopalmide® both as a single active and conveyed in a cosmetic product, in comparison with Anandamide, a well-known natural endocannabinoid with anti-inflammatory action. The anti-inflammaging activity of topically applied products has been assessed, after 6 hours of treatment post-irradiation, through the transcriptional modification of genes involved in the NF-κB pathway and the epigenetic pathway targeting miRs as potential biomarkers of inflammaging: miR-21, miR-126 and miR-146a. The results confirmed the anti-inflammatory action of Anandamide which inhibits NF-κB, while Isopalmide® showed its anti-inflammaging activity through the establishment of an inflammatory/anti-inflammatory balance by maintaining NF-κB inactive in the cytoplasm and active in the nucleus. The anti-inflammaging activity was shown also by the cosmetic product containing Isopalmide®.
2. Keywords: Endocannabinoids; Inflammaging; miRNAs; Reconstructed Human Epidermis
3. Introduction
Inflammation is widely known for affecting aging processes and for taking part in a lot of diseases which occur during senescence. Although in harmful situations, such as infections, inflammation has a physiological function helping the body to react to the pathogenic agents, when the inflammatory process does not solve or is too excessive, it becomes detrimental for the organism health. In the last two decades, the concept of inflammaging has emerged, originated mostly from the researches of Claudio Franceschi and his co-workers. According to their studies, inflammaging, a chronic low-grade inflammation that manifests itself primarily with aging [1], is caused by both external and internal factors but the main stressors have been identified in the self-debris of cells since, with aging, the organisms find it more difficult to eliminate their metabolism products [2]. Recent studies carried out on centenarians, who are still healthy and without major diseases despite their age, have highlighted significant levels of pro-inflammaging markers together with a high level of anti-inflammatory mediators, suggesting that “aging well” is not related to the absence of inflammation but to a favorable balance between inflammatory and anti-inflammatory factors [3].
Cutaneous senescence is a complex phenomenon due to multiple intrinsic and extrinsic factors. A pivotal role in senescence is played by epigenetics which refers to the modifications that occur in DNA leading to alterations in genomic expression (phenotype) without changes in genomic sequences (genotype). Epigenetics’ regulation involves several mechanisms such as DNA methylation, histone modifications and modulatory activity of miRNAs. About the latter, miRNAs are a class of short, endogenous, single-stranded RNAs that regulate gene expression [4]. They are involved in several processes such as immune responses, cell proliferation, cell death, inflammation, initiation and progression of tumors [5]. Many studies underline that during aging there is an increase in the level of miRNAs, which results in the activation of pathways related to inflammation and senescence, such as the NF-κB one, and addresses them as interesting bio-markers of these processes [6].
The skin has multiple ways to maintain its balance against inflammation and one of these is represented by the endocannabinoid system. Endocannabinoids are lipid molecules produced by the body in a wide array of situations: in the brain, they are largely known for their involvement in pain control [7]; in the skin, they regulate the differentiation and the production of mediators by various skin cells [8]. Studies in mice have reported the production of endocannabinoids as protective agents when an inflammatory skin disease occurs, supporting their involvement in the regulation of the cutaneous inflammation [9]. Endocannabinoids exert their anti-inflammatory activities mainly through the binding with their receptors, CB1 and CB2. The first ones are localized primarily in the central and peripheral nervous system, while the second ones are found on immune cells. In addition to these receptors, endocannabinoids exert their action also in a receptor-independent way.
In fact, Anandamide, the first natural endocannabinoid discovered, exerts an anti-inflammatory activity in a receptor-independent manner by acting directly on the NF-κB pathway [10], which is a nuclear factor with a pivotal role in the regulation of several genes involved in inflammation, in cell proliferation, tumor development, etc. Anandamide was proved to inhibit NF-κB-dependent transcription by inhibiting IKK activation, which is responsible for the phosphorylation of IκBα, the protein that maintains NF-κB inactive in the cytoplasm. Moreover, the inhibition of NF-κB was shown also by some anandamide analogues, such as arvanil and olvanil [10]. Based on this evidence, the aim of this study was to assess the anti-inflammaging activity of a new synthetic endocannabinoid, Isopalmide®, in comparison to Anandamide, and to verify the anti-inflammaging activity of a cosmetic product containing Isopalmide®, using an in vitro reconstructed Full Thickness Skin model.
On Phenion Skin (FT-Skin) an “inflammaging stress” model has been developed, mimicking a low grade of inflammation induced by UVA exposure associated to a light mechanical stress on the epidermal surface thus recapitulating both barrier function impairment that occurs during the aging process and dermal matrix damages induced by a relevant biological UVA dose.
The anti-inflammaging activity of topically applied products has been assessed through the transcriptional modification of genes involved in the NF-κB pathway and the epigenetic pathway targeting miRNAs as potential biomarkers of inflammaging: in particular miR-21, miR-126 and miR-146a are defined as inflamm-miRs since they target mRNA related to the NF-κB pathway [11].
The NF-κB pathway can be exemplified as follow (Figure 1): The transcription factor NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is a prominent mediator of inflammation which acts as a key transcriptional regulator of many genes coding for pro-inflammatory cytokines. NF-κB is a protein complex that both induces and represses gene expression by binding to discrete DNA sequences, known as κB elements, in promoters and enhancers. NF-κB complexes are retained as inactive forms in the cytoplasm by a family of inhibitory proteins known as inhibitors of NF-κB (IκBs). Activation of NF-κB typically involves the phosphorylation of IκB by the IκB kinase (IKK, CHUCK) complex, which results in IκB degradation. This releases NF-κB and allows the free translocation in the nucleus and activation of pro-inflammatory response [12].
The regulation of UVA-induced inflammation is furtherly actuated by epigenetic modulation via miRNA expression.
Some miRNAs, like miR-21 and miR-126, targeting mRNAs belonging to the NF-κB pathway, are classifiable as senescence-associated (SA-miRs) and inflammation-associated (inflamma-miRs). They target the NF-κB pathway primarily through a negative feedback loop aimed at retaining the excessive proinflammatory response induced by signaling activation [13].
miRNA pathway can be summarized as follow (Figure 2): In particular miR-21 over expression enhances the inflammatory response. Furthermore, miR-21 is able to reduce the expression of potent human anti-inflammatory molecules such as interleukin (IL)- 10 and TGF-β, while miR-126 plays an important role in the modulation of inflammatory activity by down-regulating the expression of IκBα, an important inhibitor of the NF-κB signaling. The regulatory negative loop for inflammatory pathway switches off is mediated by a miR-146 gene whose expression is mediated by NF-κB itself. By counteracting the pro-inflammatory status associated with cellular senescence, miR-146a can exert anti-inflammatory effects [14].
4. Materials and Methods
4.1 Materials
Phenion® Full Thickness Skin Model, produced by Henkel (Düsseldorf, Germany, diameter 1.3 cm), cultivated at 37 °C and 5% CO2 with ALI Medium. In this model, epidermal keratinocytes and dermal fibroblasts (from healthy donors) form a multilayered skin equivalent that resembles human skin under culture conditions. Fibroblasts are grown in a specialized stable matrix that does not contract under fibroblast traction forces. Keratinocytes are overlaid and within a few days, they develop an epidermis with clearly recognizable layers. Both the epidermis and dermis form a physiologically functional unit: the epidermis produces various markers of differentiation (CK10, filaggrin, transglutaminase, and involucrin). The epidermal-dermal junction presents basal membrane proteins (laminin and collagen IV). In the dermal compartment, de novo synthesis of elastin and fibronectin has been demonstrated. The proliferative cells of the basal layer are identified by Ki-67 staining. This commercial model (Phenion, Henkel) is validated for many applications in cosmetic efficacy and toxicology fields. For this study, the fibroblast and the keratinocytes derive from the same donor (no information about sex and age were supplied by the producer).
4.2 Methods
4.2.1 Quantitative Real-Time PCR Analysis
Quantitative analysis of gene expression by qRT-PCR was performed on total RNA extracted by RNAqueous kit (Ambion) and on miRNA extracted by mirVANA kit (Ambion) following the producer’s instructions. After retro transcription using the High Capacity kit (Invitrogen), the produced cDNA has been used for relative quantification PCR by using TaqMan Master Mix and gene assays (Invitrogen) referenced in (Table 1).
For data analysis, the relative quantification value (RQ) was accepted as significant when the gene expression is “one-fold” up (RQ>2) or down-regulated (RQ<0.5) compared to the calibrator sample (RQ=1). The internal instrument level of confidence used is 95%.
4.2.2 LDH release
A commercially available kit (Cytotoxicity Detection Kit-LDH, Roche) was used to quantify the LDH released in culture media by a colorimetric assay based on formazan salt detection (λ 492 nm with reference at 690 nm). The culture media were collected and analyzed following the producer’s instructions. An increase in the amount of dead or plasma membrane-damaged cells results in an increase of the LDH enzyme activity in the culture media, which directly correlates with the amount of formazan formed during a defined time period measuring the number of lysed cells.
4.2.3 Histo-morphological analysis
At the end of the treatment, tissues were fixed in 4% formalin in PBS. Samples were included in paraffin blocks and sections of 5 μm were obtained. Slides were stained with Haematoxylin and Eosin following internal procedures and the histological samples were analyzed under bright field microscopy (20x).
5. Experimental design
The FT-skin models were removed from the semi-solid transport medium and cultivated according to the supplier’s instructions using the ALI® culture medium. The FT-skin tissues were stressed at the epidermal level by a light mechanical stress in order to induce a keratinocyte mediated inflammatory response. Light mechanical abrasion was performed abrading the surface with Alger brush following internal standardized procedure in order to activate the inflammatory response without damaging stratum corneum. The FT-skin models were then irradiated with 12 J/cm2 UVA (positive control: UVA) and the formulations/actives were topically applied (50 µL) on the epidermal surface for 6 hours after irradiation. At the end of the exposure the FT skin models were collected and the gene expression was quantified by TaqMan assays for the following genes:
• miRNA: miR-21, miR-126, miR-146a
• RNA: IKK-α (CHUK), IKK-γ (IKBKG), NF-κB
Media were collected for toxicity evaluation by LDH release and tissue were fixed in 4% formalin for further histological evaluation.
In this study, the data of gene expression have been evaluated on the basis of internal validation which confirms a low variability of the model and a good reproducibility of irradiation protocols, as reported by M. Meloni et al Photochem. Photobiol. Sci., 2010, 9, 439-447.
6. Results and Discussion
6.1 Quantitative real-time PCR analysis results
The miRNA and RNA gene expression was measured after 6 hours treatment with the test items compared to the positive control (brushed and irradiated sample). Calibrator sample is the negative control, which is fixed at 1 in order to measure the expression of the genes. All the treated samples show an anti-inflammatory efficacy with different effects on miRNA and mRNA expression, as shown in (Figures 3 and 4).
The predictivity of the “inflammaging stress model” induced by UVA, on the FT skin model, has been assessed by gene expression analysis in stressed (positive control) samples. As shown in Figures 3 and 4 the UVA induces an upregulation of miR-21, that leads to a pro-inflammatory response by NF-κB increase. Moreover, the UVA is able to overexpress miR-126 which leads to an observed increase in NF-κB gene expression which triggers the inflammatory response.
Anandamide 0.5% induces an upregulation of miR-21 that leads to a pro-inflammatory response by NF-κB increase. At the same time, Anandamide is able to upregulate IKBKG (IKK-γ) that leads to phosphorylation and degradation of IκBα and the consequent increase of NF-κB expression. The NF-κB activation (upregulation) increases the miR-146 gene, which upon processing down-regulates the activity of different genes (IRAK1 and TRAF6) to reduce NF-κB activity, that remains inactive in the cytosol (Table 2).
By reducing miR-21 expression, Isopalmide® induces the retention of NF-κB in the cytoplasm that could lead to an anti-inflammatory response. On the other hand, through the blocking of miR-126Isopalmide® stimulates the IKK complex (IKBKG overexpression). At the considered time-point (6h treatment) Isopalmide® is able to establish an inflammatory/anti-inflammatory balance by the blocking of miR-21 and miR-126, indicating that it could have a precocious activity on the pathway (Figure 6): The Ceramage® Fluid Concentrate, which contains 0.5% of Isopalmide®, has an anti-inflammaging action mediated by IκBα inhibitory control of NF-κB.
The blocking of miR-21 induces the levels of NF-κB in the cytoplasm (inactive form), resulting in an anti-inflammatory response. The missing of the IKK complex activation (IKBKG and CHUK not significant expression) prevents the phosphorylation of IκBα: the consequence is an increase of NF-κB in the cytosol as an inactive form, resulting in an anti-inflammatory activity of Ceramage® Fluid. The behavior of Ceramage® Fluid Concentrate can be represented as follow (Figure 7).
6.2 LDH release and histological analysis
The results of the LDH release, after 6 hours of treatment post-irradiation, are shown in (Figure 8).
Lactate dehydrogenase release in the culture medium was measured to quantify the membrane integrity. This enzyme is normally present in the cytosol and cannot be measured extracellularly unless cell damage has occurred. So, the release of LDH reflects the membrane damage and correlates to a toxic effect. Considering the value quantified in the control as the basal LDH release, an increased (3.04 fold increase) was observed in UVA-irradiated tissues suggesting the induction of the inflammaging model. After 6 hours of treatment the products compared to UVA-irradiated tissues induces the following results: Anandamide induces a decrease of LDH release (49% reduction of LDH release), reflecting its anti-inflammatory action; Isopalmide® at 0,1% does not show a difference in LDH release compared UVA tissues (6% decrease); Isopalmide® at 0.5% shows a lower LDH release compared to UVA tissues (28% of reduction); Ceramage® Fluid Concentrate, which contains Isopalmide® at 0.5%, induces a decrease of LDH release (57% reduction) reflecting its anti-inflammatory action on the whole tissue (dermis/epidermis).
These results were also confirmed by the histology of the tissues treated with tested items for 6 hours post-irradiation, as shown in (Figure 9).
The morphology of the positive UVA-irradiated control appears severely modified on the dermal compartment (papillary upper dermis) indicating that the target of the inflammaging regulates skin architecture. At the epidermal level, the overall morphology appears not significantly modified.
In the sample treated with Anandamide 0.5%, the extracellular matrix of the papillary dermis appears restored and there are no signs of damage at the tissue level, confirming its marked anti-inflammatory activity. The tissue treated with Ceramage® Fluid concentrate that contains Isopalmide® 0.5% appears even more restored, suggesting both an increase of the bioavailability of Isopalmide® by the vehicle and an additional role of other components of the cosmetic product.
7. Conclusions
The aim of the study was to assess the activity of a new synthetic endocannabinoid, Isopalmide®, in comparison to a natural well-known endocannabinoid, anandamide. We tested the activity of both compounds on an in vitro skin model, previously validated for the expression of inflammation after irradiation with UVA and we evaluated the activity of anandamide and Isopalmide® on the NF-κB pathway, considering that in literature the effects of anandamide on this pathway have already been assessed [10]. Given these assumptions, we observed that anandamide and Isopalmide® act on the NF-κB pathway in a different way: the first inhibits NF-κB activation through a negative regulatory feedback promoted by miR-146a, while the second establish an inflammaging/anti-inflammaging balance by the blocking of miRNA-21 and miRNA-126 upregulation. Moreover, we tested on the same model a cosmetic product containing Isopalmide® at 0.5% and we observed an anti-inflammaging activity by preventing the activation of NF-κB through IKB-α inhibitory control and by reducing of the inflammatory miRNAs.
8. Patents
Isopalmide® is a patented molecule (EP1900365). Isopalmide® and Ceramage® Fluid Concentrate are registered trademarks of Unifarco S.p.A.
9. Author contributions
Marisa Meloni and Alessandra Semenzato conceived and designed the experiments; Elisa Caviola performed the experiments; Marisa Meloni and Elisa Caviola analyzed the data; Gianni Baratto provided the materials (endocannabinoids and cosmetic products); Giulia Galizia wrote the paper.
10. Conflicts of interest
The following study was sponsored by Unifarco S.p.A. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.

Figure 1: Schematic representation of the NF-kB pathway
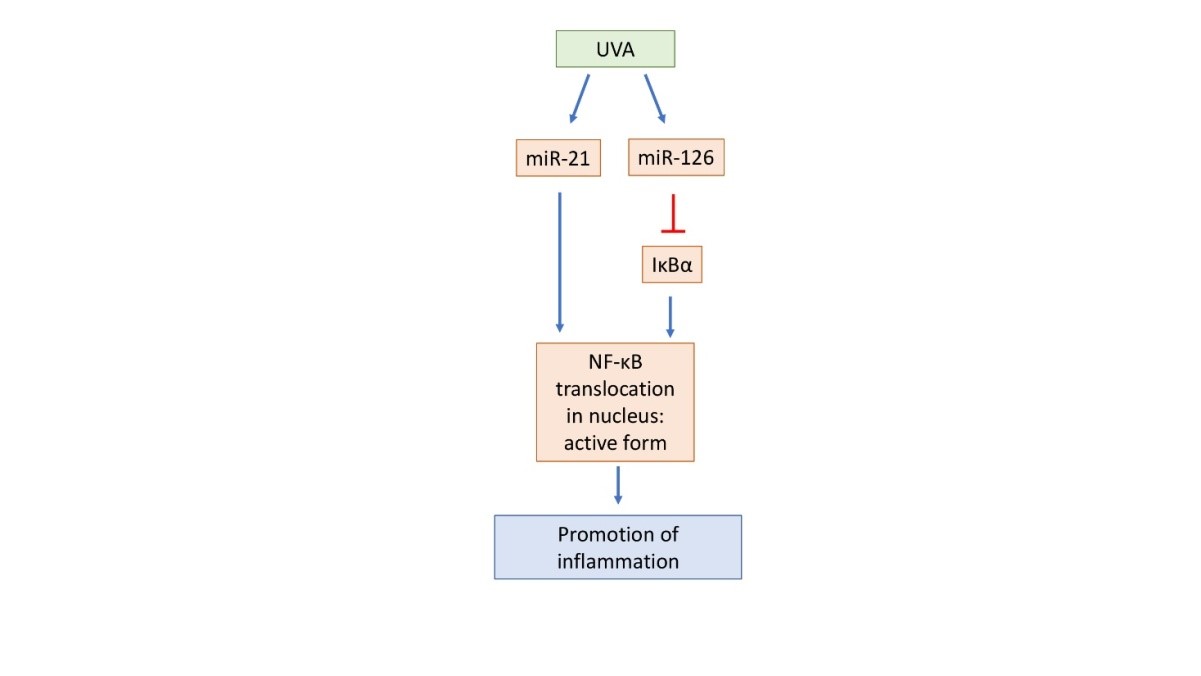
Figure 2: Schematic representation of the miRNA pathway. The blue arrows indicate an upregulation, while the red arrows indicate a down regulation
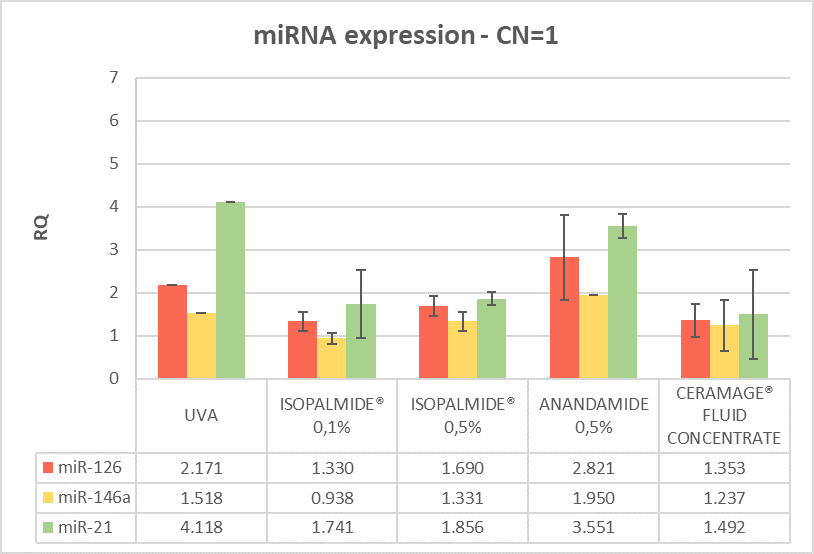
(Legend: RQ=1 Negative control, RQ ≤ 0.5 significant down regulation, RQ ≥ 2 significant up regulation)
Figure 3: miRNA gene modulation after 6 hours treatment post-irradiation
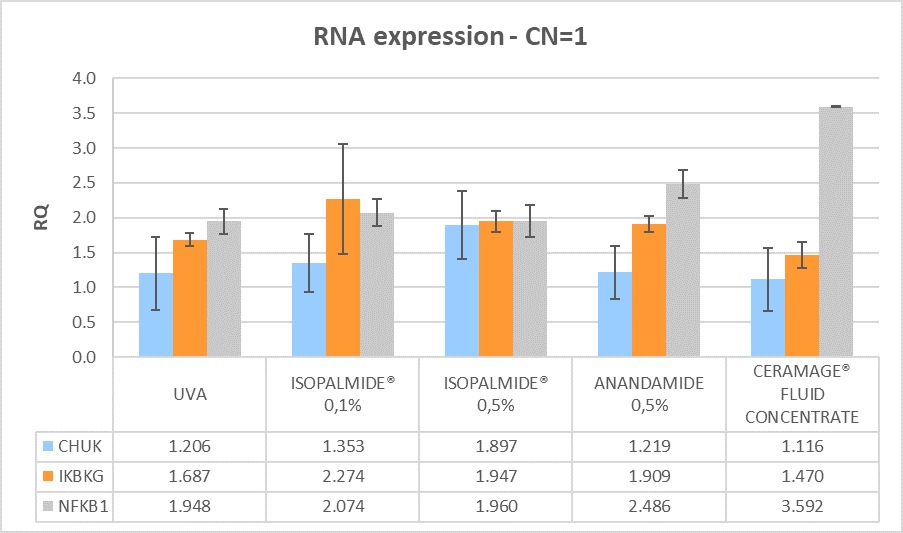
(Legend: RQ=1 Negative control, RQ ≤ 0.5 significant down regulation, RQ ≥ 2 significant up regulation)
Figure 4: mRNA gene modulation after 6 hours treatment post-irradiation
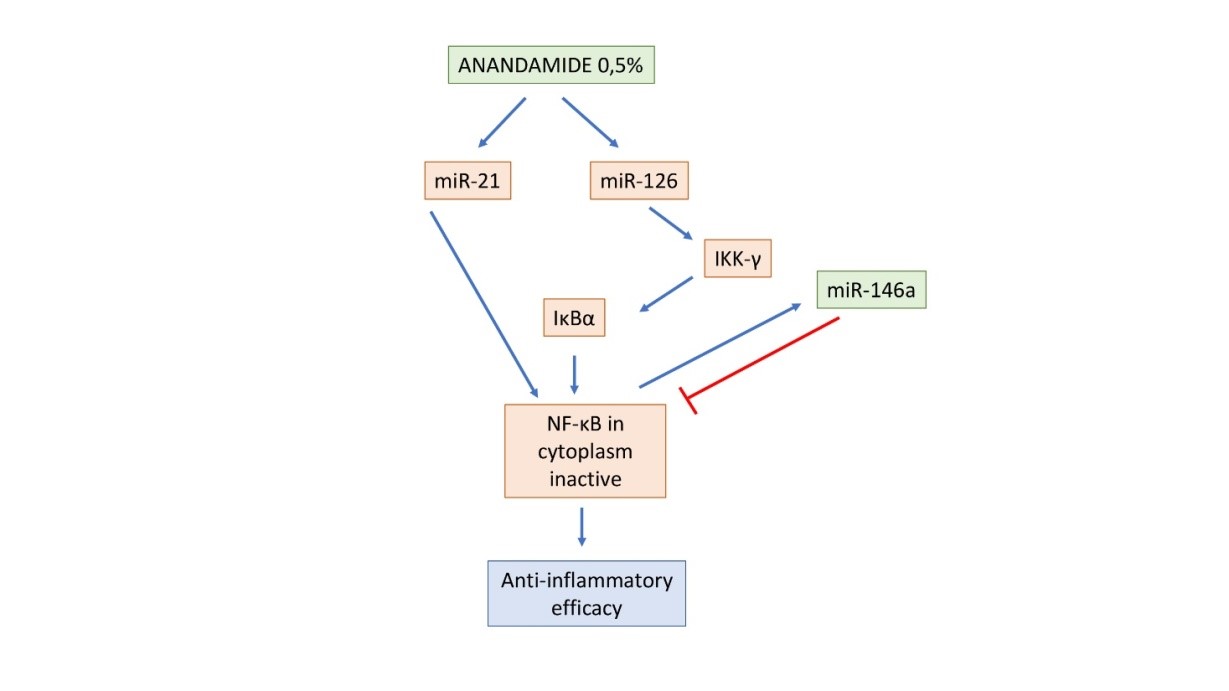
Figure 5: Schematic representation of the suggested mechanism of action of Anandamide 0.5% on miRNAs and NF-κB expression. The blue arrows indicate an up regulation, while the red arrows indicate a down regulation.
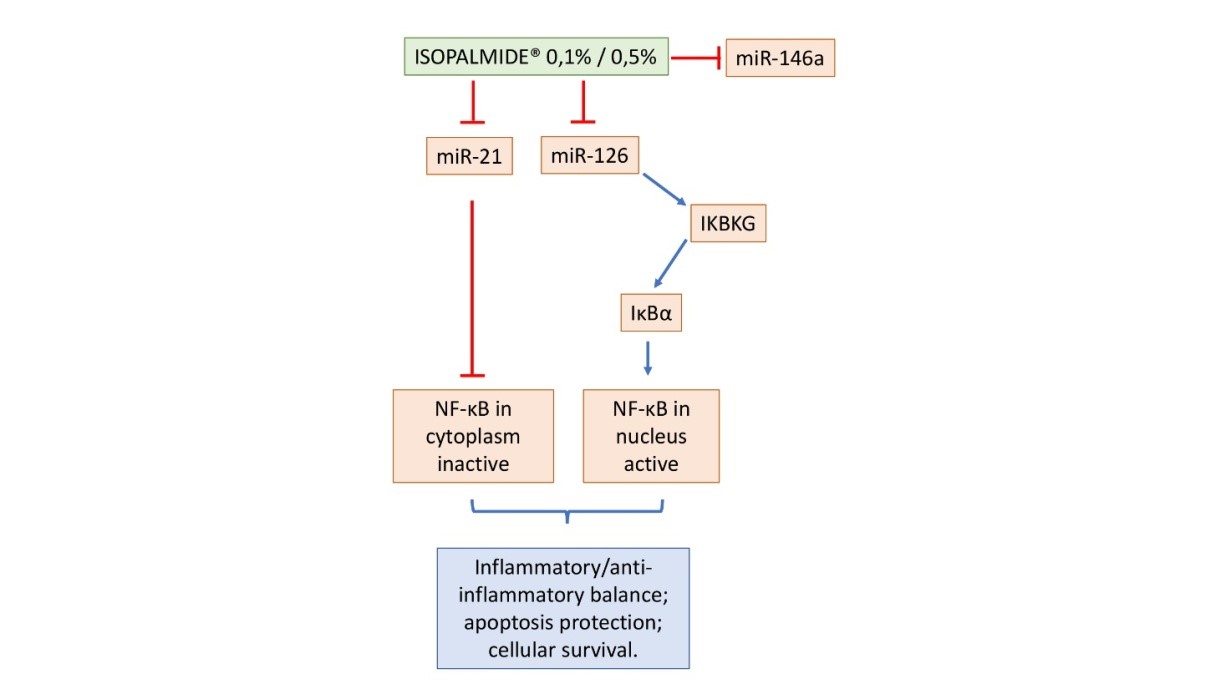
Figure 6: Schematic representation of the suggested mechanism of action of Isopalmide® 0.1% and 0.5% on miRNAs and NF-κB expression. The blue arrows indicate an up regulation, while the red arrows indicate a down regulation
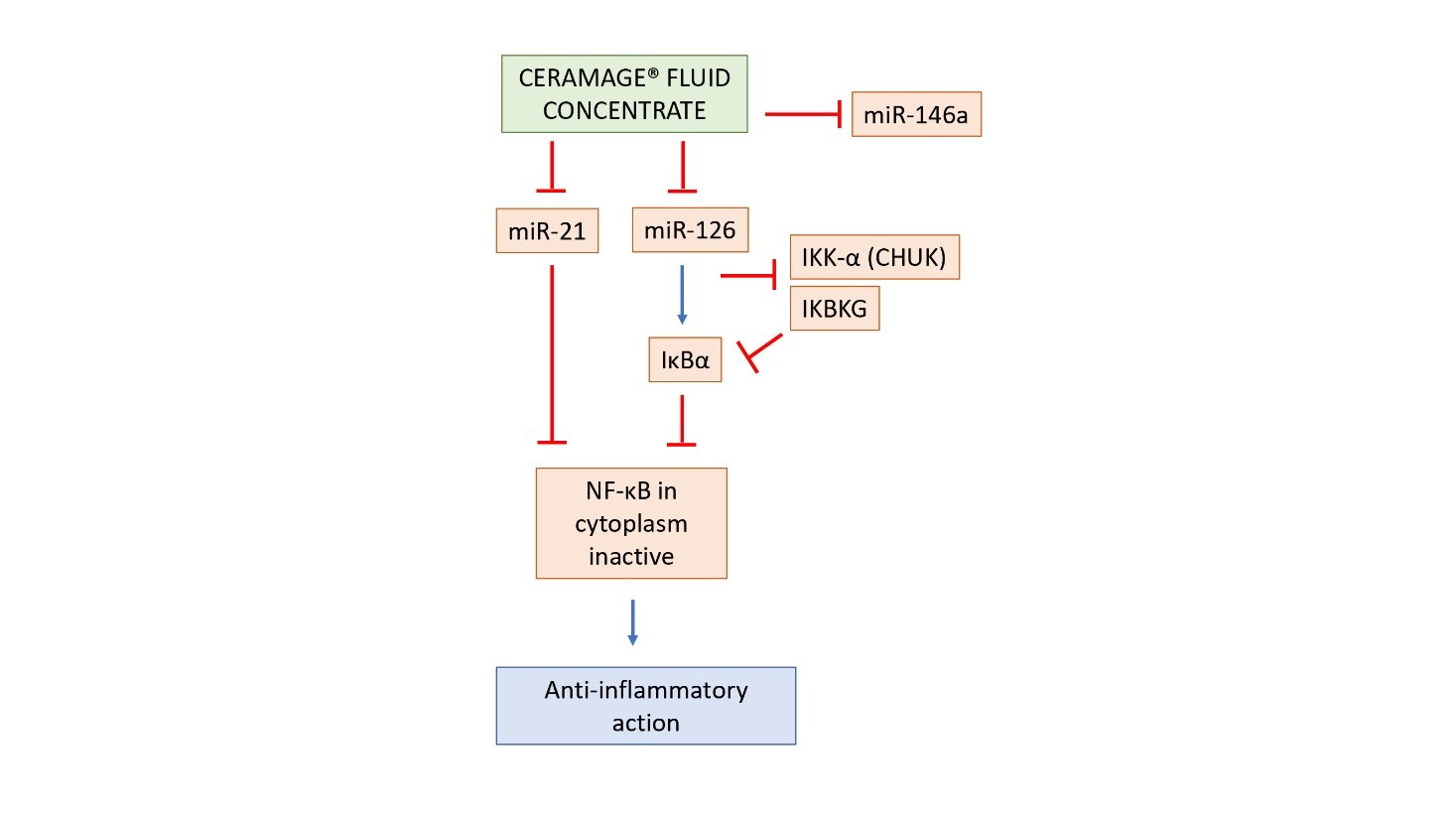
Figure 7: Schematic representation of the suggested mechanism of action of Ceramage® Fluid Concentrate on miRNAs and NF-κB expression. The blue arrows indicate an up regulation, while the red arrows indicate a down regulation
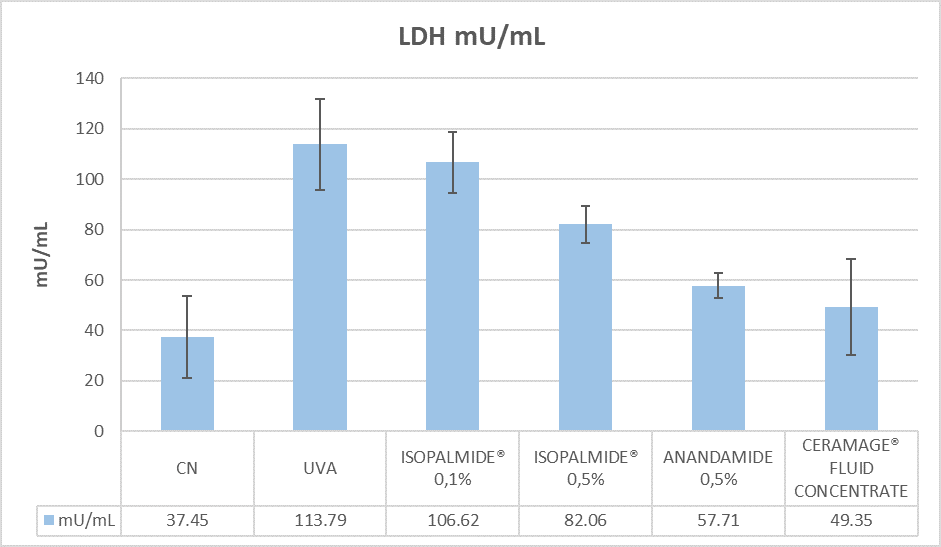
Figure 8: LDH release in the culture media of collected samples after 6 hours of treatment with tested compounds
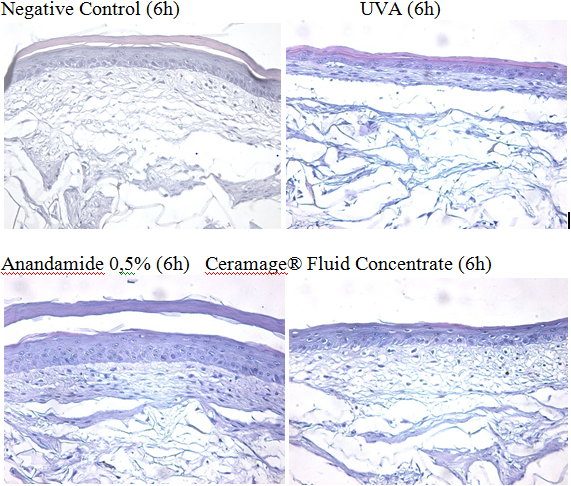
Figure 9: H&E staining histology after 6 hours treatment post-irradiation analysed under light microscopy (20x)
|
miRNA |
Code |
|
HSA-miR-21 |
P161018-005 H01 |
|
HSA-miR-126 |
P161005-020 F10 |
|
HSA-miR-146a |
P160919-006 C04 |
|
U18 |
P160831-000 H11 |
|
Genes (RNA) |
Code |
|
IKK-α (CHUK) |
Hs00989502_m1 |
|
NF-κB |
Hs00765730_m1 |
|
GADPH |
Hs99999905_m1 |
Table 1: TaqMan assays used in the qRT-PCR analysis
|
|
miR-126 |
miR-146a |
miR-21 |
CHUK |
IKBKG |
NFKB1 |
|
UVA |
2.171 ± 0.000 |
1.518 ± 0.000 |
4.118 ± 0.000 |
1.206 ± 0.524 |
1.687 ± 0.089 |
1.948 ± 0.174 |
|
Anandamide 0.5% |
2.821 ± 0.986 |
1.950 ± 0.000 |
3.551 ± 0.291 |
1.219 ± 0.379 |
1.909 ± 0.115 |
2.486 ± 0.196 |
|
Legend: RQ=1 Negative control, RQ ≤ 0.5 significant down regulation, RQ ≥ 2 significant up regulation |
||||||
Table 2: miRNA and RNA gene expression after irradiation and treatment with Anandamide 0.5%
|
|
miR-126 |
miR-146a |
miR-21 |
CHUK |
IKBKG |
NFKB1 |
|
UVA |
2.171 ± 0.000 |
1.518 ± 0.000 |
4.118 ± 0.000 |
1.206 ± 0.524 |
1.687 ± 0.089 |
1.948 ± 0.174 |
|
Isopalmide®0,1% |
1.330 ± 0.219 |
0.938 ± 0.130 |
1.741 ± 0.796 |
1.353 ± 0.415 |
2.274 ± 0.787 |
2.074 ± 0.198 |
|
Legend: RQ=1 Negative control, RQ ≤ 0.5 significant down regulation, RQ ≥ 2 significant up regulation |
||||||
Table 3: miRNA and RNA gene expression after irradiation and treatment with Isopalmide® 0.1%
|
|
miR-126 |
miR-146a |
miR-21 |
CHUK |
IKBKG |
NFKB1 |
|
UVA |
2.171 ± 0.000 |
1.518 ± 0.000 |
4.118 ± 0.000 |
1.206 ± 0.524 |
1.687 ± 0.089 |
1.948 ± 0.174 |
|
Isopalmide®0,5% |
1.690 ± 0.231 |
1.331 ± 0.222 |
1.856 ± 0.152 |
1.897 ± 0.487 |
1.947 ± 0.156 |
1.960 ± 0.229 |
|
Legend: RQ=1 Negative control, RQ ≤ 0.5 significant down regulation, RQ ≥ 2 significant up regulation |
||||||
Table 4: miRNA and RNA gene expression after irradiation and treatment with Isopalmide® 0.5%
|
|
miR-126 |
miR-146a |
miR-21 |
CHUK |
IKBKG |
NFKB1 |
|
UVA |
2.171 ± 0.000 |
1.518 ± 0.000 |
4.118 ± 0.000 |
1.206 ± 0.524 |
1.687 ± 0.089 |
1.948 ± 0.174 |
|
Ceramage® Fluid Concentrate |
1.353 ± 0.384 |
1.237 ± 0.598 |
1.492 ± 1.045 |
1.116 ± 0.450 |
1.470 ± 0.185 |
3.592 ± 0.009 |
|
Legend: RQ=1 Negative control, RQ ≤ 0.5 significant down regulation, RQ ≥ 2 significant up regulation |
||||||
Table 5: miRNA and RNA gene expression after irradiation and treatment with Ceramage® Fluid Concentrate
Citation: Semenzato A, Meloni M, Caviola E, Galizia G and Baratto G (2018) A New Synthetic Endocannabinoid as Anti-Inflammaging Cosmetic Active: an In Vitro Study on a Reconstructed Skin Model. Curr Updates Dermatol Probl: CUDP-100003.