Journal of Obstetrics and Gynecological Problems
(ISSN 2652-466X)
Research Article
The New Concept of Cervical Cancer Screening and Follow-Up through the COVID-19 Pandemic
Kang YN1†, Zhang Y1*, Tian Y1, Liou YL2,3*, Ma Y4†, Fang C5,6 and Tao H7
1Department of Obstetrics and Gynecology, Xiangya Hospital, P. R. China
2Department of Clinical Pharmacology, Xiangya Hospital, Central South University, P. R. China
3Institute of Clinical Pharmacology, Central South University, Hunan Key Laboratory of Pharmacogenetics, P. R. China
4The First Affiliated Hospital of University of South China, P. R. China
5Department of Anesthesiology, Third Xiangya Hospital of Central South University, P. R. China
6Postdoctoral Research Workstation of Clinical Medicine, The Third Xiangya Hospital, Central South University, China
7Hunan Hoomya Gene Technology Co., Ltd. China
Equal contributors
*Corresponding authors: 1.Yu Zhang: cszhangyu@126.com; Department of Obstetrics and Gynecology, Xiangya Hospital, Central South University, Hunan 410008, People’s Republic of China
2.Yuligh Liou, Department of Clinical Pharmacology, Xiangya Hospital, Central South University, China.
Citation: Kang YN, Zhang Y, Tian Y, Liou YL, Ma Y, et al. (2020) The New Concept of Cervical Cancer Screening and Follow-Up through the COVID-19 Pandemic. J ObstetGynecolProbl: JOGP 100012
Received date: 20 June, 2020; Accepted date: 30 June, 2020; Published date: 06 July, 2020
Abstract
Objective: To recommend the new concept of cervical cancer diagnosis and management in hospital by evaluating the efficacy of
different screening methods.
Methods: The clinical data and samples of 155 women were collected and analysis in Xiangya Hospital of Central South Universi- tyfrom January 2018 to May 2020. Liquid based cytology (LBC), high-risk HPV, and TruScreen (TS01) were used to evaluate the effectiveness.The positive rate, sensitivity and specificity of cytology, hrHPV, colposcopy, and TS01 were calculated at the CIN2+ cut off point of pathological results.
Results: The positive result of TS01 in normal, CIN1, CIN2 and CIN3+ lesions, the positive rates of TS01 were 23.3%, 63.4%, 80.0%, and 85.7% respectively. The positive rate of hrHPV, cytology, and colposcopy at CIN2 and worse were 100%, 79.2%, and 95.8%. The positive rate of hrHPV and cytology with inflammation and normal cervix pathologic results were 75.6% and 46.7%, respectively. The gynecological department of Xiangya Hospital set up a new patient management process during the COVID-19 pandemic, the closed- loop disease monitor and treatment management system has done the great effect during COVID-19 pandemic period.
Conclusion: The new cervical cancer screening and follow-up management concept to reduce the coronavirus infection and precision therapy by the optical and electrical instrument (TruScreen) and the closed-loop disease monitor and treatment management system was study and recommended in the study.
Keywords: Cervical cancer; Cytology; COVID-19 pandemic; hrHPV; TruScreen
Introduction
Coronavirus disease (COVID-19) epidemic has challenged global health and rapidly spread over many countries in these 5 months. June 17, 2020, it had resulted 8,061,550 infections and 440,290 deaths worldwide[1]. In China, 83,293 COVID-19 infection confirmed cases and 4,634 deaths were reported [2]. In Hunan, the closely Province to Wuhan in China, had 1,019 infection cases and 4 cases died [2]. The COVID-19 epidemic has caused severe psychological pressure specially the medical staff in most clinics and hospitals.
General hospital services have faced severe challenge because of the shortage of beds, hands, and prevention supplies. In order to control the spread of COVID-19 and cure the serious events of high-risk patients, especially the management of cancer, it is particularly important to implement public health measures and telemedicine services. According to a recently literature, patients with cancer had a higher risk of developing severe events (intensive care unit admission, invasive ventilation, or death) compared with patients without cancer (39 % vs 8%, p=0·0003) [3].
In terms of gynecological cancer, FRANCOGYN group for the CNGOF propose recommendations that it is not too different from the standard of care, but they emphasize radio-chemotherapy at the front line of cervical cancer instead of immediate surgical treatment.To change the treatment procedures and management of cervical cancer in order to reduce the infection risk of medical staff by surgery and the management burden caused by long-term ICU hospitalization [4]. The similar proposal was made by a group of multinational practitioners[5]. Cervical cancer development takes a long time in most patients, in particular from precancer to invasive cancer. High-grade cervical intraepithelial neoplasia (CIN2 and CIN3) can develop within 3-5 years after high-risk human papilloma virus (hrHPV) infection, and progression to invasive cancer may take 20-30 years[6-8]. This long period of time provides many opportunities for intervention and has probably contributed to the success of frequent cervical cancer screening to reduce the incidence and mortality of cervical cancer in the developing countries. The long-term development characteristics of cervical precancerous lesions also provide many management and follow-up possibilities compared with other cancers during the COVID-19 epidemic.
The artificial intelligence has been widely applied in the medical field, such as cytology, histopathology, imaging and other important areas [9,10]. The new generation of cervical cancer screening system (TruScreen, TS01) is a new portable instrument which can use optical and electrical signals to analyze cervical tissues with a built-in algorithm in real time. The device was introduced in The Cervix (2nd ed) for use in cervical cancer screening without specimen collection[11].
The purpose of this study is to evaluate the sensitivity and specificity of TS01, noninvasive, fast and simple used, by comparing with the existing liquid-based cytology (LBC) and hrHPVtesting for cervical cancer diagnosis. The other purpose of this study is to evaluate the possible risk among the cytology, hrHPV, and TS01 in the detection of cervical lesions in hospitals.
Materials and Methods
Patient recruitment: A total of 155 women visited the gynecology clinics were invited to the studies in Xiangya hospital from January 2018 to May 2020. The average age of the patients was 39.7 years (23-77 years). The inclusion criteria: women with sexual life over 20 years old, who voluntarily participated in and signed the informed consent form.Exclusive criteria: Patients had history of cervical cancer treatment or pregnant women. After cytology and hrHPVsample collection, the patients were transferred to colposcopy according to indications. TheTS01 and colposcopy were performed in colposcopy room.
Cytological diagnoses: According to instruction, samples of cervical exfoliate cells were collected using a cytology brush and stored in the Cyto Fast solution tube (Hospitex Diagnostics SRL, Sesto Fiorentino, Italy). The cytology results were classified according to the 2014 Bethesda System (TBS 2011). The results of reports are no intraepithelial lesion or malignancy finding (NILM), atypical squamous cells of undetermined significance (ASC-US), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), cannot exclude high-grade squamous intraepithelial lesion (ASC-H), atypical glandular cells (AGC), squamous cell carcinoma (SCC), and adenocarcinoma (AD).
HPV DNA testing: Cobas 4800 (Roche, Basel, Swiss) was used for hrHPV genotyping. HPV16 type, HPV18 type and other 12 type of hrHPVwere test and reported by Cobas 4800. All laboratorial procedures were performed according to the recommendations of the reagent instruction.
TruScreen examination: The TS01 was performed by gynecologists in colposcopic clinic in the study. The TS01 diagnosis was performed before colposcopic examination. The women lay down in lithotomy position, after inserting speculum, the operator disposes the cervix entirely, then inserts the handpiece that has been mounted the Single Use Sensor (SUS) into the vagina. Following the probing pattern set in the operational manual the surface of the cervix is probed by the tip of the handpiece point by point. The TS01 result will be printed out after the whole cervix detected and then press the button on the touch screen of the handpiece. The TS01 is reported as normal or abnormalresults.
Colposcopy and histopathology: A standardized colposcopic examination of the cervix was performed. The colposcopic examination was performed by qualified gynecologists by Colposcopy and Cervical Neoplasia Committee of China (CCNC). Each examination was performed with 3-5% acetic acid and Schiller test. Lesions were described in terms of color, margin, vessels, and iodine-staining characteristics.The finial pathologic result of subject was determined at the most serious one in biopsy, ionization, or operative tissue in the study.
Results
The closed-loop disease monitor and treatment management system: The gynecological department of Xiangya hospital set up a new patient management process during the COVID-19 pandemic, the closed-loop disease monitor and treatment management system (Figure 1).
After patients registered their name on this management system, the medical records, diagnosis reports, and image records could upload to the system. The first line gynecologic physicians of the system could evaluate the patients whether going to take the next step diagnosis or treatment after reviewing the information from the management system. Patients could discuss with physicians by the telemedicine of the management system to know go clinic or hospitalization. After the patient is discharged from Xiangya hospital, the patient could also check and testing by order from the system in the nearby hospital and send the data to this system for follow-up management.
Characteristics of subjects and colposcopy biopsy results: A total of 155patients were collected and analyzed in Table 1.
The average age was 38.92 years (20-88 years), of which 58.1% were normal inflammation (90/155), 40.0% (62/155) of cervical intraepithelial lesion (CIN), 2.0% (3/155) of cervical cancer. The positive rate of TS01, ASCUS+, and hrHPV were 43.2% (67/155), 58.7% (91/155), and 84.5% (131/155) (Table 2).
The positive result of TS01 in normal, CIN1, CIN2 and CIN3+ lesions, the positive rates of TS01 were 23.3%, 63.4%, 80.0%, and 85.7% respectively. The positive rate of hrHPV, cytology, and colposcopy at CIN2 and worse were 100%, 79.2%, and 95.8%. The positive rate of hrHPV and cytology with inflammation and normal cervix pathologic results were 75.6% and 46.7%, respectively.
Sensitivity and specificity of tests for CIN2+ by analysis of each testing: The sensitivity and specificity of eachdiagnosis testing were summary at Table 3. For detection CIN2+, the sensitivity and specificity of TS01 was 83.3% and 64.1%, respectively. The highest sensitivity was 100.0% of hrHPV test but with lower specificity 18.3%. Colposcopy was 95.8% sensitivity at detection CIN2+ in the study.
Discussion
Although some recommendations suggested to distinguish the people by positive or negative coronavirus testing results or high or low body temperature before entering the hospital, only refer to the patients with “possible or positive diagnosis of COVID-19” patients to hospital. If patients were negative results or lower body temperature, it should be noted that, depending on the test or temperature detection, the false negative rate of these tests may vary between 15% and 25% [12]. A safer principle to consider the new management system is to limit access and time between doctors and patients.
During the period of epidemic prevention, all the patients were managed by the closed-loop disease monitor and treatment management system. The gynecological ward of Xiangya hospital mainly treats the following three kinds of patients: (1) gynecological emergency; (2) gynecological tumor patients who need operation within a time limit; (3) gynecological tumor patients who need regular chemotherapy. After more than five months’ management following the new system, we can provide the best medical treatment for the real-time high-risk patients and avoid the possible risk of coronavirus infection between patients and medical staffs in the hospital. Up to now, no any COVID-19 infection has happened in the gynecologic patients and staff teams. Most of the literatures emphasize ward management and operation management, but few literatures focus on the outpatient management or specific disease management[13-15].
We hope to find out the possible high sensitivity and high specificity of cervical cancer screening or diagnosis methods in hand, as well as the shortest patients and specimens contact time with medical staff in the hospital, as recommendations in the COVID-19 epidemic period.
Cytology is a widely used screening method for cervical cancer in clinical practice, but the results are different by subjective factors and the experience of cytologists. The sensitivity of cytology is about 30%~87% [16]. In the study, the performance of cytology is 79.2% but the high percentage of ASC, including ASC-US and ASC-H, were approximately high. Due to the lack of experienced cytologists in local hospitals or laboratories, the cytological based cervical cancer diagnosis method could not be the first choice of follow-up methods fitting the Xiangya telemedicine management system. The large number of ASC patients referred for colposcopy examination and follow-up could be an extra burden and risk for physicians during the colposcopy examination in the COVID-19 pandemic period.
The American Cancer Society (ACS) and the American Society for Colposcopy and Cervical Pathology (ASCCP) recommend that either co-testing every 5 years or cytology-only screening every 3 years should be implemented for women aged 30-65 years [16]. American Society of Colposcopy and Cervical Pathology (ASCCP) announced the latest cervical cancer screening interim clinical guidance, advocated hrHPV primary screening program in 2015 [17]. However, cervical cancer cells develop to precancerous lesions via HPV infection and become invasive cancer for several years or even decades. 4%~10% develops into HPV persistent infection, and HPV positive patients have a clearance rate of 87.65% more than 1 year [18]. The high sensitivity but less specific HPV testing may lead to over-colposcopicreferral rate, patient panic, overtreatment and too many visits of hospitals. Therefore, the huge number of hrHPV patients visits of hospitals and referred for colposcopic examinationcould bean extra burden and high risk for patients and medical staff.
TS01 is a sensor device for optical and electrical analysis of cervical tissue in order to detect precancerous lesions and cancerous lesions. When the characteristics of the cervical epithelial cells change, the sensor was detected the change of the light reflection in the cervical lesion tissue [19].TS01 can detect not only the surface epithelium of the cervix, but also the cervical epithelium.The special frequency of light can also be transferred to the cervical tissue to detect changes in the blood vessels of the subcutaneous layer of the cervix. Many literatures reported that TS01 was easy used for opportunistic screening, follow-up, cervical cancer screening specially in the areas of lack of resources [20]. The new generation of TS01 is simpler and easier to operate than the first generation of TS. The results of this study show that the TS01 (new generation of TS) has 83.3% sensitivity and 64.1% specificity, which is more sensitive than the original TS 76% (95%CI: 73%~80%) and specificity 69% (95% CI: 67%~71%) [21]. The advantage of TS01 not be reported before was no extra medical staffs contact the specimens and patients between departments in hospital. The possible risk limited in the outpatient physician for TS01 operation (less than 5 minutes for all process). The risk of possible infection by people to people could be reduced by TS01 in the hospital. For example, many staffs and risk were involved in the cytology report processincluding the specimen collection, specimen transportation to pathology department, specimen reception, cell centrifugation, cell fixation, cell staining, reading the slid, and reporting. All the time and man power were summary at Table 4.
The novel coronavirus, also known as COVID-19 has become a worldwide threat and healthcare concern. Human to human transmission of the virus occurs through respiratory droplets and through direct contact with an infected patient or indirect contact in his environment. Coronavirus is still a very new for us, so less information and timing for us to develop effective prevention process. During the COVID-19 epidemic period, it is the first paper to discuss the cervical cancer screening and follow- up management concept.
The main purpose of the cervical cancer specially for cervical pre-cancerous management concept is to ensure the treatment will not be delayed by the COVID-19 pandemic. It is more important to reduce the coronavirus contact risk when the patient in the way to hospital and in hospitals. Moreover, it is also an important consideration of this concept to reduce the risk of medical staff’s contact with patients and their specimens at work. In the study, TruScreen and the closed-loop disease monitor and treatment management system could really solve the problem of diagnosis and treatment of cervical cancer in the epidemics period.
In addition, the 155 patient samples were collected during colposcopy because the majority of patients with obvious cervical cancer underwent biopsy immediately following abnormal Pap smear test results and clinical observation in the outpatient department. The selected patients by guidance made the limitation reflecting not all the patients. Other limitations include the small sample size and a lack of extensive and long-term follow-up information.
Conclusion
The new cervical cancer screening and follow-up manage- ment concept to reduce the coronavirus infectionand precision therapy for patients by the optical and electrical instrument (Tru- Screen) and the closed-loop disease monitor and treatment man- agement system was study and summary in the study.
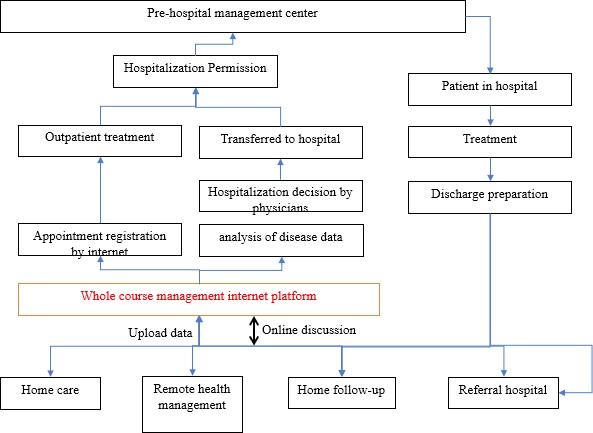
Figure 1: The closed-loop disease monitor and treatment management system.
|
Index |
Number(n) |
Percent (%) |
|
Age (total number n=155) |
||
|
mean (range) |
39.7 (23~77) |
|
|
<30 |
36 |
23.20% |
|
30~50 |
90 |
58.10% |
|
≥50 |
29 |
18.70% |
|
Pathology |
||
|
SCC |
3 |
1.90% |
|
CIN 3 |
11 |
7.10% |
|
CIN 2 |
10 |
6.50% |
|
CIN1 |
41 |
26.5 |
|
Normal or inflammation |
90 |
58.10% |
|
Cytology (LBC) |
||
|
SCC or HSIL |
4 |
2.60% |
|
LSIL |
14 |
9.00% |
|
ASC-H |
7 |
4.50% |
|
ASCUS |
66 |
42.6 |
|
NILM |
64 |
41.3 |
|
High-risk HPV (hrHPV) |
||
|
positive |
131 |
84.5 |
|
negative |
24 |
15.50% |
|
TruScreen (TS01) |
||
|
Abnormal |
67 |
43.20% |
|
normal |
88 |
56.80% |
|
Note: SCC, squamous cell carcinoma; AC, adenocarcinoma; CIN, cervical intraepithelial neoplasia; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; ASC-H, atypical squamous cells, cannot exclude a high-grade lesion; ASCUS, atypical squamous cells of undetermined significance; NILM, negative for intraepithelial lesion or malignancy; hrHPV, high-risk human papillomavirus. |
||
Table 1: The basic information of the women in the study.
|
Tests |
Normal/ inflammation |
CIN1 |
CIN2 |
CIN3 |
Cervical cancer |
|
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
|
|
Subjects |
90(100.0) |
41(100.0) |
10(100.0) |
11(100.0) |
3(100.0) |
|
TS01 |
21(23.3) |
26(63.4) |
8(80.0) |
9(81.8) |
3(100.0) |
|
LBC(≥ASCUS) |
42(46.7) |
30(72.3) |
5(50.0) |
11(100%) |
3(100.0) |
|
hrHPV(+) |
68(75.6) |
39(95.1) |
10(100.0) |
11(100%) |
3(100.0) |
|
Colposcopy impression (≥LSIL)
|
42(46.7) |
33(80.5) |
9(90.0) |
11(100%) |
3(100.0) |
|
Note: CIN, cervical intraepithelial neoplasia; LBC, liquid-based cytology; hrHPV, high-risk human papillomavirus; n, number; LSIL, low-grade squamous intraepithelial lesion; ASCUS, atypical squamous cells of undetermined significance; TS01, TruScreen. |
|||||
Table 2: The positive rate of different tests.
|
Tests |
Cut off value |
Sensitivity (95%CI) |
Specificity (95%CI) |
OR Value (95%CI) |
P Value |
|
TS01 |
Abnormal |
83.3%(61.8-94.5) |
64.1%(55.2-72.2) |
8.94(2.88-27.70) |
P<0.001 |
|
LBC |
≥ASCUS |
79.2%(57.3-92.1) |
45.0%(36.4-54.0) |
3.114(1.10-8.84) |
p=0.033 |
|
hrHPV |
HR-HPV(+) |
100%(82.8-100) |
18.3%(12.3-26.2) |
11.17(0.66-190.03) |
P=0.095 |
|
Colposcopy impression |
≥LSIL |
95.8%(76.9-99.8) |
42.7%(34.2-51.7) |
17.17(2.25-131.00)
|
P=0.006 |
|
Note: CIN, cervical intraepithelial neoplasia; LBC, liquid-based cytology; hrHPV, high-risk human papillomavirus; n, number; LSIL, low-grade squamous intraepithelial lesion; ASCUS, atypical squamous cells of undetermined significance; TS01, TruScreen. |
|||||
Table 3: Performance of three diagnosis tests for identifying women with CIN2+.
|
Diagnosis method |
Sampling |
Sampling time |
Sample isolation |
Number of staffs involved |
The time from sampling to report |
|
hrHPV |
√ |
~ 2 mins |
high |
4 |
3 hrs |
|
Conventional cytology |
√ |
~ 2 mins |
low |
4 |
1 hr |
|
Liquid-based cytology |
√ |
~ 2 mins |
high |
4 |
2 hrs |
|
Colposcopic examination |
√ |
~15 mins |
high |
2 |
30 mins |
|
TruScreen |
No need |
~ 5 mins |
No need |
1 |
10 secs |
|
Note:hrHPV, high-risk human papillomavirus; TS01, TruScreen; min, minute; hrs, hours; sec, second. |
|||||
Table 4: The summary of time and process of different tests.
Citation: Kang YN, Zhang Y, Tian Y, Liou YL, Ma Y, et al. (2020) The New Concept of Cervical Cancer Screening and Follow-Up through the COVID-19 Pandemic. J ObstetGynecolProbl: JOGP 100012