Journal of Diabetes Management and Metabolism
ISSN 2652-4430
Review Article
Translating the HemoglobinA1c Assay into Estimated Average Glucose Values-A New Term to Replace HbA1c
Ali IA*
Department of Physiology, Faculty of Medicine,The National Ribat University
*Corresponding author: Ibrahim Abdelrhim Ali, Department of Physiology, Faculty of Medicine,The National Ribat University, Sudan.
Citation: Ali IA (2020) Translating the Hemoglobin A1c Assay into Estimated Average Glucose Values-A New Term to Replace Hb A1c. J Diabetes ManagMetab: JDMM-10010
Received date: 15 May, 2020; Accepted date: 06 June, 2020; Published date:12 June, 2020
Abstract
Glycated Hb (Hb A1c) has been accepted as the gold standard measurement for the assessment of chronic hyperglycaemia for nearly three decades. There are thirty different laboratory methods available to measure glycated hemoglobin.
After three decades from using Hb A1c, new researches and trials were conducted to find out the relationship between average blood glucose and glycatedhaemoglobin, and a linear regression equation was developedto measure average blood glucose from Hb A1c.
The relationship between HbA1C and average blood glucose is complex but has been studied by the Diabetes Control and Complications Trial (DCCT).
A new internationally standardized method for reporting HbA1C has been developed by the International Federation of Clinical Chemistry (IFCC), they recommended the useof a new unit, i.e. mmol Hb A1c /mol of total haemoglobin instead of the percentage.
Meanwhile byusing theequation can calculate the average blood glucose from glycated haemoglobin in mmol/mol.
This average blood glucose will be reported as “eAG” (estimated average glucose) and it will be used to monitor glucose control as eGFR (estimated glomerular filtration rate) is used to monitor renal function in chronic kidney disease patients.
Keywords: Estimated average glucose; eAG; HbA ; Glycated Hb; International Federation of Clinical Chemistry (IFCC)
Introduction
Standardization of Glycated Haemoglobin Hb A1c measurement: In the 1980s and 1990s an important issue that concerned Hb A1c measurement was the lack of standardization of the assay, and this meant that different analysers could have widely differing reference intervals and yield varying results with patient samples[1].Standardization of HbA1cmeasurement has been proposed in different countries to ensure accuracy in HbA1c results [2].
Glycated Hb has been accepted as the gold standard measurement for the assessment of chronic hyperglycaemia for
nearly three decades. There are thirty different laboratory methods available to measure glycated hemoglobin. Various analytical laboratory methods based on different assays principles, from ion- exchange chromatography to immunoassay and electrophoresis have been used to measure glycated hemoglobin. Such a lack of standardization resulted in wide variability and debates within results (4.0% to 8.1%) on the same sample making it difficult to compare patient’s results among laboratories [3].
This disparity has always been a source of anxiety among health care providers. It becomes even more important in this age of heavy economical migration, when people travel long distances and take their native record with them. Therefore, having the same method and unit to measure Hb A1c is very critical need.
In 2007, the IFCC recommended that Hb A1cresults can be expressed as mmolHbA1c instead of Hb A1c percentage. Patients
using mmol/l or mg/dl for self-monitoring of day-to-day glucose control find it difficult to understand when their doctors discussed hemoglobin levels in percentages. To eliminate confusion and streamline these discrepancies, a consensus statementon the worldwide standardization of HbA1c measurement was adopted in May 2007 by the ADA, European Association for the Study of DiabetesEASD, IDF and IFCC. It states that new IFCC reference system is the only valid anchor for implementing the standardization of the measurement of Hb A1c [4].
Since 1stJune 2009, Hb A results in the UK have been standardized to the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) which will allow global comparison of results, with the equivalent normal non-diabetic range of IFCC-Hb A1c being 20-42 mmol/mol.
It was also resolved that if the on-going “average plasma glucose study” was concluded successfully (i.e. confirmed the relationship between average blood glucose and Hb A1c) then the A1c. Derived Average Glucose Equivalent would also be reported as an interpretation of Hb A1c results[4].
Assays for Hb A1c use technologies based on either charge differences high performance liquid chromatography (HPLC) or structure (boronate affinity or immunoassay combined with general chemistry) and newly techniques based on combined immunoassay and general chemistry[5].
In past decades, the trials such as DCCT (Diabetes Control and Complications Trial) and UKPDS (UK Prospective Diabetes Study) considered Hb A as the gold standard of diabetes care [2,6,7].
Furthermore, the programs such as NGSP (National Glycohemoglobin Standardization Program) and associations such as AACC (American Association for Clinical Chemistry) (Kahn & Fonseca, 2004)[8] have worked on standardization of Hb A1cvalues leading to present methods in measurement of Hb A1c. Recently, NGSP certifies rapid Hb A1cassays have become available, allowing office and home testing[7].
Switch to IFCC units
After three decades from using Hb A1c, new researches and trials were conducted to find out the relationship between average blood glucose and glycated haemoglobin, and a linear regression equation was developed to measure average blood glucose from Hb A1c.
The relationship between HbA1C and average blood glucose is complex but has been studied by the Diabetes Control and Complications Trial (DCCT).A new internationally standardized method for reporting HbA1C has been developed by the International Federation of Clinical Chemistry (IFCC), they recommended the use of a new unit, i.e. mmolHb A1c /mol of total haemoglobin instead of the percentage [4].Meanwhile by using the equation can calculate the average blood glucose from glycated haemoglobin in mmol/mol.
This average blood glucose will be reported as “eAG” (estimated average glucose) and it will be used to monitor glucose control as eGFR (estimated glomerular filtration rate) is used to monitor renal function in chronic kidney disease patients. The A1c -derived average glucose study was conducted in 10 different locations in North America, Europe, and Africa. The goal of the study was to report glycated haemoglobin results not in the usual Hb A1c percentage format but as A1c derived averages in the same units used in self-monitoring, (i.e., mg/dl or mmol/l). The study concluded that the estimated average glucose (eAG) can now be calculated from Hb A1c using a linear regression equation[8,9].
The American Diabetes Association (ADA), European Association for the Study of Diabetes (EASD) and International Diabetes Federation (IDF) have agreed that, in the future, HbA1c is to be reported in the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) units[10].IFCC reporting was introduced in Europe except for the UK in 2003;[11]the UK carried out dual reporting from 1 June 2009 [12]until 1 October 2011(Table 1-5).
Conversion between DCCT and IFCC is by the following equation [12].

in DCCT percentage (%) and eAG (estimated average glucose) measurements is given by the following equation:[9].
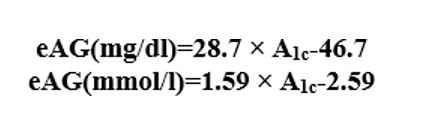
Data in parentheses are 95% confidence intervals
|
DCCT- Hb A1c (%) |
IFCC- Hb A1c (mmol/mol) |
|
3.1 |
10 |
|
4 |
20 |
|
4.9 |
30 |
|
5.8 |
40 |
|
6 |
42 |
|
6.3 |
45 |
|
6.5 |
48 |
|
6.7 |
50 |
|
7 |
53 |
|
7.2 |
55 |
|
7.5 |
59 |
|
7.6 |
60 |
|
8 |
64 |
|
8.1 |
65 |
|
8.6 |
70 |
|
9 |
75 |
|
9.5 |
80 |
|
10.4 |
90 |
|
11.3 |
100 |
Table 1: Comparing DCCT- Hb A1c and IFCC- Hb A1c Results. The approximate mapping between HbA1c values given
|
HbA1c % |
mmol/ mol |
mmol/L |
mg/dL |
|
5 |
31 |
5.4 (4.2-6.7) |
97 (76-120) |
|
6 |
42 |
7.0 (5.5-8.5) |
126 (100-152) |
|
7 |
53 |
8.6 (6.8-10.3) |
154 (123-185) |
|
8 |
64 |
10.2 (8.1-12.1) |
183 (147-217) |
|
9 |
75 |
11.8 (9.4-13.9) |
212 (170-249) |
|
10 |
86 |
13.4 (10.7-15.7) |
240 (193-282) |
|
11 |
97 |
14.9 (12.0-17.5) |
269 (217-314) |
|
12 |
108 |
16.5 (13.3-19.3) |
298 (240-347) |
|
13 |
119 |
18.1 (15-21) |
326 (260-380) |
|
14 |
130 |
19.7 (16-23) |
355 (290-410) |
|
15 |
140 |
21.3 (17-25) |
384 (310-440) |
|
16 |
151 |
22.9 (19-26) |
413 (330-480) |
|
17 |
162 |
24.5 (20-28) |
441 (460-510) |
|
18 |
173 |
26.1 (21-30) |
470 (380-540) |
|
19 |
184 |
27.7 (23-32) |
499 (410-570) |
Table 2: Show HbA1c % corresponded to different laboratory values.
|
NGSP |
IFCC |
eAG |
eAG |
|
HbA1c (%) |
HbA1c (mmol/mol) |
(mg/dL) |
(mmol/l) |
|
4 |
20 |
68 |
3.8 |
|
6 |
42 |
126 |
7 |
|
7 |
53 |
154 |
8.6 |
|
8 |
64 |
183 |
10.2 |
|
9 |
75 |
212 |
11.8 |
|
10 |
86 |
240 |
13.4 |
|
11 |
97 |
269 |
14.9 |
|
12 |
108 |
298 |
16.5 |
Table 3: Show mapping between HbA1c values given in DCCT percentage (%) and eAG (estimated average glucose) measurements.
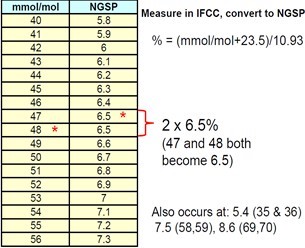
Table 4:Show Conversion from IFCC into NGSP [9].
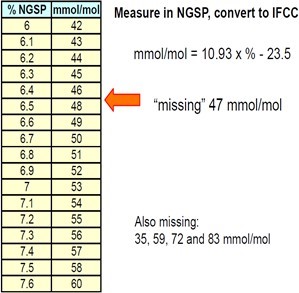
Table 5:Show Conversion from NGSP into IFCC [9].
Kahn R and Fonseca V (2008) Translating the A1c assay. Diabetes Care. 31: 1-4.
Nathan DM, Kuenen J, Borg R (2008) Translating the A1c Assay into Estimated Average Glucose Values. Diabetes Care 31: 1473-8.
Geistanger A, Arends S, Berding C, Hoshino T, Jeppsson JO, et al. (2008)Statistical methods for monitoring the relationship between the IFCC reference measurement procedure for hemoglobin A1c and the designated comparison methods in the United States, Japan, and Sweden. Clin. Chem 54: 1379-1385.
Manley S, John WG, Marshall S (July 2004) Introduction of IFCC refer- ence method for calibration of A1c implications for clinical care. Diabet. Med 21: 673-676.
The Association for Clinical Biochemistry and Diabetss UK: Standardi- sation of the reference method for the measurement of Hb A1c to im- prove diabetes care.
Citation: Ali IA (2020) Translating the Hemoglobin A1c Assay into Estimated Average Glucose Values-A New Term to Replace Hb A1c. J Diabetes ManagMetab: JDMM-10010