Emerging Infectious Diseases and Diagnosis Journal
ISSN 2652-4449
Review Article
Targeting COVID-19 Inflammation and Oxidative Stress
Moss RB* and Carlo DJ
Adamis Pharmaceuticals Corporation, San Diego, USA
*Corresponding author:Ronald B. Moss, Adamis Pharmaceuticals, San Diego, USA
Citation:Moss RB and Carlo DJ (2020) Targeting COVID-19 Inflammation and Oxidative Stress.Emerg Infect Dis Diag J: EIDDJ-100025
Received date: 16 June, 2020; Accepted date: 19 June, 2020; Published date: 22 June, 2020
Abstract
The Current COVID19 pandemic has proven to be a public health crisis and a serious challenge to the medical community. The novel respiratory infection results in significant morbidity and mortality. The lungs are a primary target organ for virus associated pathology often resulting in acute respiratory distress syndrome. Dysfunctional inflammatory cascades, as well as oxidative stress, play important roles in the pathogenesis of tissue damage due to CIVID19 infection. Targeting the inflammation and oxidative stress pathways with therapeutics agents may be critical medical countermeasures in limiting morbidity and mortality from COVID19.
Keywords: Anti-inflammatory; Antioxidants, IL-6, Tempol; Cytokines; COVID-19; Hydroxychloroquine; JAK inhibitors; Reactive oxygen species
Introduction
On December 12, 2019, 27 cases of viral pneumonia were reported at Wuhan Municipal Health Commission in China[1]. The novel virus was first identified using a reverse transcription polymerase chain reaction (PCR) on a broncho alveolar sample from an ill pneumonia patient who tested positive for pan-beta corona virus (WHO, 2020). Further sequencing of the virus revealed a novel virus now called SARS-CoV-2, and whose clinical manifestations are known as COVID-19[2]. On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a global pandemic (WHO, 2020). Because of the virus is novel there is no relevant pre-existing protective immunity.
COVID-19 belongs to the beta-coronavirus cluster. COVID-19, the disease caused by SARS-CoV-2, is the third known zoonotic coronavirus following Severe Acute Respiratory Syndrome (SARS-CoV-1) and Middle East Respiratory Syndrome (MERS). COVID-19 has close sequence homology to SARS-CoV-1 (about 80%)[3]. The Angiotensin-Converting Enzyme-2 (ACE2) receptor is the receptor for both COVID-19 and SARS-CoV-1[4]. ACE2 is expressed on cell surfaces of multiple tissues including the alveoli type 1 and 2 cells in the pulmonary epithelia, heart tissue, blood vessels and kidneys[2]. Therefore, dysregulated inflammatory responses and oxidative stress related to COVID-19 can manifest itself with pathology in many different organ types.
The mortality and morbidity from COVID-19 continues to mount, as does our understanding of the pathogenesis of the disease. The disease has distinguished itself by recent evidence of multi-organ system inflammation and oxidative stress. This paper will review what is known about COVID-19 inflammation and oxidative stress and then will follow with a discussion on potential treatment modalities.
Target Organ Lungs: ARDS
The lungs are the main target organ for COVID-19. In a meta-analysis of 38 studies in 3,062 patients with COVID-19 infection[5], cough (63.1%) was among the most common clinical manifestation followed by chest tightness (35.7%), shortness of breath (35%), and dyspnea (33.9%). Radiographically, 75.7% of patients had lesions involving bilateral lungs and 25.8% of patients had lesions involving a single lung lobe.Of note, 19.5% of the patients had acute respiratory distress syndrome (ARDS).
The end-stage process for many patients with COVID-19 is ARDS. There are various definitions of ARDS.The Berlin definition of ARDS requires that onset occur within 7 days of clinical insult, or new or worsening respiratory symptoms. Also, there should be evidence of bilateral lung opacities that are consistent with pulmonary edema on chest CT or radiograph. Furthermore, the Berlin definition characterizes the severity of ARDS based on the ratio of decreased arterial blood oxygen tension (Pa02) to fraction of inspired oxygen (Fi02) ratio[6]. ARDS, in general, can occur because of pneumonia, sepsis or trauma. Most recently ARDS was observed with other respiratory viruses including pandemic H1N1 influenza[7] SARS and MERS[8].
The immunopathogenesis of ARDS is incompletely understood. However, both a dysregulated innate immune response as well as oxidative stress can result in damage to the alveoli of the lungs with accumulation of edema within the alveoli and interstitial. Macrophages, neutrophils and endothelial cells are thought to play a key role with secretion of multiple inflammatory cytokines including TNFalpha, IL-1B, IL8, IL6, CCL7 and CCL2 [9]. The cytokines released from macrophages induce additional cell recruitment including neutrophils, monocytes, and effector T cells. NF-kappa B is a rapidly inducible transcription factor that plays a major role in activating inflammatory pathways in ARDS.In addition, there is endothelial activation resulting in barrier disruption, which then results in pulmonary edema leading to lung function abnormalities. Complementing this cascade of cytokines is the release of reactive oxygen species due to oxidative stress. This will be discussed later. This process of dysfunctional immune activation, which is not limited to the lungs, has been referred to as the “Cytokine Storm”[10].To date, there are no effective treatments for ARDS[11].The complex involvement of inflammatory cytokines and oxidative stress releasing ROS in ARDS is depicted in the alveoli of the lungs in Figure 1.
Reactive Oxygen Species (ROS) in ARDS
During oxidative stress, the activation of phagocytes such as macrophages and neutrophils infiltrate the pulmonary circulation and cause the release of free radicals and cytotoxic reactive oxygen species (ROS), as well as multiple cytokines.Examples of ROS that are free radicals which can cause tissue damage are superoxide (O2-) and hydroxyl (OH-). A number of stimuli can activate reactive oxygen species including TNF-alpha, platelet activating factor and endotoxin. ROS combined with decreased antioxidant activity results in significant cellular damage in the lungs, including damage to the endothelial and epithelial cells.Furthermore, the production of large amounts of superoxide by activated phagocytes can result in a cascade releasing cytokines like TNF- alpha and IL1. In totality, release of ROS as well as inflammatory cytokines causes significant lung injury, resulting in increased alveolar and pulmonary edema.Ultimately, severe hypoxia results in multi-organ failure and death.
Systemic immune markers of inflammation observed with COVID-19
Numerous studies have documented high levels of a broad number of inflammatory markers in the blood of patients with COVID-19. In one series, patients with COVID-19 pneumonia were found to have high systemic levels of C-reactive protein, erythrocyte sedimentation rate, and IL-6[11]. In another series, patients requiring the intensive care unit (13 ICU versus 28 non-ICU) had higher systemic levels of IL-2, IL-10, GM- CSF, MCP-1, MIP-1 alpha, TNF-alpha, and chemokines compared to non-ICU patients[12]. However, a number of other systemic cytokines were elevated comparing the ICU patients to healthy normal, including IL-1b, IL1ra, IL-2,IL-4,IL-8,IL-7,IL-8,IL-9,IL-10,IL-13,IL-17,
FGF-basic, GM-CSF, Gamma interferon,IP-10, MCP-1, MIP-1 alpha, PDFG, MIP-1 Beta, TNF-1 alpha, and VEGF. Thus, the cytokine storm observed with COVID-19 includes a broad array of cytokines from macrophages, neutrophils, and T cells. In addition, release of ROS, as discussed before, is involved in the cytokine storm causing damage to the target organ.
An interesting study examining the predictive use of systemic inflammatory cytokines suggested that systemic IL-6 levels can be predictive of the patients who require mechanical ventilation. Elevated interleukin-6 (IL-6) was strongly associated with the need for mechanical ventilation.
Target Organ - the heart:As mentioned earlier, the receptor of COVID-19 is ACE2. In animal models ACE2 is an essential regulator of the heart.Cardiac involvement in COVID-19 is prevalent and some myocardial injury biomarkers such as troponin and Brain-type natriuretic peptides are common, and patients with higher markers are at higher risk of death[13]. It is possible that inflammatory cytokines and oxidative stress plays a role in cardiac and other tissue damage in COVID-19.
Anti-inflammatory and antioxidant drugs for the treatment of COVID-19
A number of treatments including antivirals, antibodies, hyper immune globulin, and anti-inflammatory drugs are being investigated for the treatment of COVID-19. To date, no drugs have been shown to be safe and effective in treating the inflammatory component of COVID-19(NIH Treatment Guidelines 2020). However, the US National Institutes of Health (NIH press release 2020) has announced positive treatment effect on the clinical data time to recovery for COVID-19 patients from a large clinical trial of Remdesivir (NIH 2020). These results conflict with a smaller trial which showed no clinical benefit of the drug[14].
In an attempt to target inflammation and oxidative stress, several approaches are being tested for COVID-19. These include the use of corticosteroids, anti-cytokine inhibitors, JAK inhibitors, hydroxychloroquine, and antioxidants as shown in Figure 2.
Corticosteroids:Glucocorticoids were used in the treatment of influenza pneumonia, SARS-Cov-1 and MERS. However, some studies failed to show any clinical benefit, but rather adverse events and delayed viral clearance[15-20]. Currently, glucocorticoids are being used to treat COVID-19 patients, Multiple randomized studies are ongoing to examine the preventative and treatment role of glucocorticoids in COVID-19 infection.
Anti-cytokines: Many different anti-cytokine antibodies are in development for the treatment of COVID-19. One prototype cytokine is IL-6 inhibitors. IL-6 inhibitors have been used for the treatment of rheumatoid arthritis. One such inhibitor, tociluzumab, is a recombinant human IL-6 monoclonal which binds to soluble and membrane bound IL-6 receptors, thereby blocking IL-6 mediated inflammatory responses[21-25].Multiple randomized trials of tociluzumab and other IL-6 inhibitors are ongoing with no results as of this publication. A single center study of 15 patients from China revealed no beneficial effects of tociluzumab in COVID-19 patients[26]. Clinicaltrials.gov lists many trials of tociluzumab and other anti-cytokines for the treatment of COVID-19 at the time of this manuscript.
JAK inhibitors:JAK inhibitors work by inhibiting the activity of one or more of the JAK family of enzymes. The JAK family of enzymes are responsible for signal transduction and JAK inhibitors play a major role in inhibiting and blocking cytokine release that can contribute to inflammation and growth of malignant cells. JAK inhibitors are used in the treatment of cancer and inflammatory diseases such as rheumatoid arthritis[27].One JAK inhibitor, Baricitinib, is being studied in randomized clinical trials for COVID-19[28].Baricitinib may reduce both the viral entry and the inflammation in 2019-nCoV patients. ClinicalTrials.gov lists many trials of JAK inhibitors for the treatment of COVID-19.
Hydroxychloroquine/Chloroquine:Hydroxychloroquine (HQ)/chloroquine(Q) is a frontline medication for the treatment and prophylaxis of malaria and is also used to treated systemic lupus erythematous and rheumatoid arthritis. These medications have in-vitro antiviral activities by interfering with attachment of the viral particles to their receptor or interfering with the pH-dependent endosome mediated viral entry[29]. Although the mechanism of action in autoimmune diseases is unknown, it is possible that HQ/Q effects cell signaling and decreases the production of pro-inflammatory cytokines. A small initial study showed some activity of hydroxychloroquine in combination with azithromycin[30],but toxicity was observed in other studies including heart arrythmias at higher doses[31].There are multiple ongoing trials for HQ/Q for the treatment of COVID-19.
Antioxidants: Oxidative stress and reactive oxygen species play an important role in tissue and lung damage associated with ARDS. Therefore, agents which serve as antioxidants may have a beneficial effect in limiting reactive oxygen species in COVID-19 disease. One interesting antioxidant drug which has been tested for its ability to protect against radiation induced reactive oxygen species is Tempol. In addition to its anti-inflammatory activity, Tempol works catalytically and acts as an antioxidant or superoxide dismutase mimetic[32].Tempol appears to down regulate NF-kappaB, a key regulator of inflammation[33]. In animal models of acute and chronic lung injury, Tempol preserved lung architectures and decreased multiple inflammatory cytokines[34]. Tempol is currently being investigated as a treatment for COVID-19.
Conclusion
COVID-19 has resulted in significant morbidity and mortality due to the incomplete understanding of the pathogenesis of this novel virus. Dysfunctional Inflammation and oxidative stress most likely contribute to the pathological process and therefore present rational targets to treat and prevent the tissue damage and the destruction that characterizes the clinical sequalae. Until a safe and effective preventive vaccine becomes available, targeting drugs that limit inflammation and oxidative stress may result in safe and effective therapies that limit morbidity and mortality from COVID-19.
Competing Interests: RBM and DJC are employees of Adamis Pharmaceuticals. Adamis Pharmaceuticals is developing Tempol for the treatment of COVID-19.

Figure 1: In ARDS, release of inflammatory cytokines and ROS result in injury to the epithelium and endothelium in the alveoli resulting in hypoxia and further stimulation of additional cytotoxic agents.
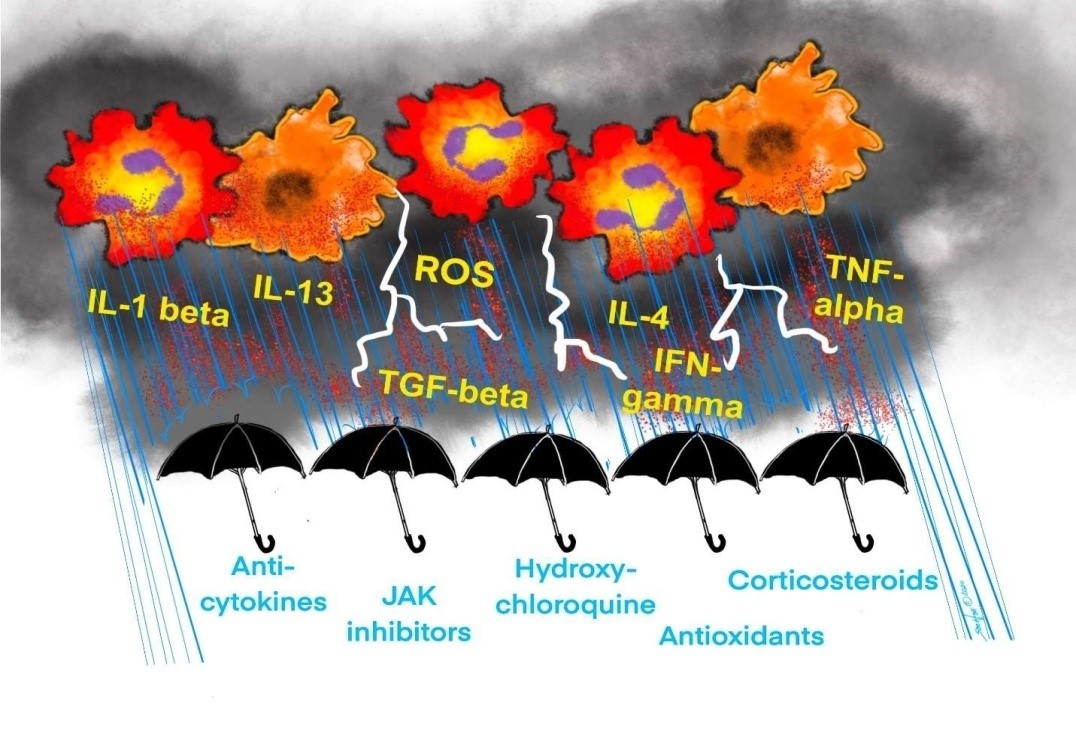
Figure 2:Various therapeutic modalities are being attempted to treat cytokine storm and ROS release, including Anti-cytokines, JAK inhibitors, Hydroxychloroquine, Corticosteroids, and Antioxidants.
Citation:Moss RB and Carlo DJ (2020) Targeting COVID-19 Inflammation and Oxidative Stress.Emerg Infect Dis Diag J: EIDDJ-100025