Annals of Medical & Surgical Case Reports
ISSN 2652-4414
Case Report
Management of Corona Virus Disease 2019 in a Patient with a Congenital Long-QT Syndrome: A Case Report
Al-Ghamdi B1,2*, Echahidi MN1, Edathodu J3 and Alrajhi A2,4
1Department of Heart Centre, Riyadh, King Faisal Specialist Hospital & Research Center.
2Alfaisal University, Saudi Arabia
3Department of Internal Medicine, Riyadh, King Faisal Specialist Hospital & Research Center, Alfaisal University, Saudi Arabia
4Chief Educationand Training Officer, Education & Training, King Faisal Specialist Hospital & Research Center, Alfaisal University,
Saudi Arabia
*Corresponding author: Bandar Al-Ghamdi, Department of Heart Centre, Riyadh, King Faisal Specialist Hospital & Research Center, Alfaisal University, Saudi Arabia
Citation: Al-Ghamdi B, Echahidi MN, Edathodu J, Alrajhi A (2020) Management of Corona Virus Disease 2019 in a Patient with a Congenital Long-QT Syndrome: A Case Report. Ann Med & Surg Case Rep: AMSCR-100066
Received date: 02 June, 2020; Accepted date: 20 June, 2020; Published date: 26 June, 2020
Abstract
A 27-year-old female was admitted with a confirmed corona virus disease 2019 (COVID-19). She was known to have con- genital long QT syndrome (LQTS) with recurrent syncope. She had implantable cardioverter-defibrillator (ICD) implantation a few years ago. She was treated with hydroxychloroquine and antiviral drugs. She had further QT prolongation on treatment but without significant untoward clinical sequelae.
Keywords: Arrhythmia; COVID-19; Chloroquine; Hydroxychloroquine; Long QT syndrome; Torsades de pointe
Abbreviations
AV : Atrioventricular
COVID-19 : Coronavirus Disease 2019
HIV : Human Immune Deficiency Virus
LQTS : Long QT Syndrome
SARS-CoV or SARS-CoV : Severe Acute Respiratory Syndrome Coronavirus
SARS-CoV-2 : Severe Acute Respiratory Syndrome Coronavirus 2
TdP : Torsade de Pointes
Introduction
The pandemic of coronavirus disease 2019 (COVID-19) has started in late 2019 and resulted in over 4.5 million cases and more than 300,000 fatalities by mid-May 2020. Currently, there are no specific approved medications for the treatment of COVID-19, but there are trials of using existing antiviral and other drugs.
A small study in France showed that hydroxychloroquine alone or, combined with azithromycin, shortened the time to resolution of viral shedding of COVID-19 [1]. However, chloroquine and its derivative hydroxychloroquine and azithromycin and some antiviral drugs may prolong the QT interval with a potential risk of ventricular arrhythmia and sudden death[2,3]. Baseline QTc>500 milliseconds (ms) (or >530-550 ms in patients with QRS greater than >120 ms) and the presence of a history of a long-QT syndrome are considered relative contraindications to use these medications [4].
We are presenting a case of COVID-19 with a congenital long-QT syndrome (LQTS) treated with hydroxychloroquine and antiviral drugswithout significant untoward clinical sequelae.
Case Report
A 27-year-old female presented to the Emergency Department (ED) on 18 March 2020 with a two-day history of fever, runny nose, back pain, and malaise. She has a history of contact with a febrile person and a person returning from East Asia. She reported palpitations with occasional fast heartbeats for a short period for two weeks.
She was known to have congenital LQTS with recurrent syncope. She had undergone implantable cardioverter-defibrillator (ICD) implantation, and she was on bisoprolol 5 mg daily. She had no family history of sudden cardiac death or LQTS. Genetic testing for LQTS was negative.
On presentation to the ED, she had a temperature of 37.9 degrees Celsius (°C), blood pressure 104/68 mmHg, heart rate 75 beats per minute, respiratory rate 20 per minute, and Oxygen saturation 98% on room air. Cardiovascular, pulmonary, and systematic examinations were normal.
Laboratory test; White blood cells (WBCs) 3.72 × 109/Liter (L), Hemoglobin 126 gram/L (g/L), and platelets 186× 109/L. Lymphocyte count was low at 18.3% (normal range 23-60%). Renal profile and liver enzymes were normal. The cardiac enzymes were normal.
The nasopharyngeal swab was positive for COVID-19 using a real-time reverse transcription-polymerase chain reaction (rRT-PCR). Screening for influenza A and B viruses with its subtypes, parainfluenza, respiratory syncytial virus, adenovirus, and other coronavirus were negative. C-reactive protein was 2.2 mg/L, D-Dimer 0.55 microgram/ mL (μg/mL) (0.00-0.50), and ferritin 37.6 μg/L (13-150).
Electrocardiogram (ECG) showed sinus rhythm with a QTc interval of 454 ms. Her old ECGs showed QTc of 500 ms. The QTc was measured in Lead II using the maximum slope-intercept method and Fridericia’s formula.
The chest X-ray was normal. Also, her echocardiogram was normal.
She was admitted to an airborne and contact isolation room. She was started on Oseltamivir 75 milligram (mg) orally twice daily, and intravenous (IV) Ceftriaxone 2 gm daily in ED. Hydroxychloroquine and azithromycin were not considered initially because of the history of long-QTc and history of QTc of about 500 ms. Her Tisdale risk score using two QTc-prolonging drugs was nine, putting her at the upper limit of moderate risk [5].
She complained of nausea and vomited once. There was no abdominal pain or diarrhea.
Despite the initial improvement in her temperature with paracetamol as needed, she spiked temperature at 38.1°C and had shortness of breath. A high resolution computed tomography (CT) of the chest was performed, and it showed normal lungs. Oseltamivir was discontinued, and she was started on Ribavirin 2000 mg daily, which was discontinued one day later because of a drop in hemoglobin by 2 g/L. She was also started on Lopinavir-Ritonavir (400 mg and 100 mg) orally twice daily.
The managing team felt that hydroxychloroquine would be a better option for her based on the new limited data on hydroxychloroquine use in COVID-19. She was started on hydroxychloroquine with continuous telemetry monitoring and daily ECGs. Hydroxychloroquine was given as 400 mg twice daily for one day and then 200 mg twice daily for five days. Potassium level was maintained close to 5.0 mmol/l and magnesium level above 1.0 mmol/L. ECG showed increased QTc to 529 ms after 24 hours of starting hydroxychloroquine, but it stayed the same with no arrhythmias and no ICD therapy (Figure 1 a,b). She completed the five days’ course of hydroxychloroquine and lopinavir-ritonavir successfully. The nasopharyngeal swab was repeated twice on 4 and 5 April 2020, and it was negative for COVID-19. The ICD analysis did not record any ventricular arrhythmia. She was discharged home in stable condition.
Discussion
Currently, there is no specific treatment of COVID-19 patients but empirically chloroquine and hydroxychloroquine and anti-viral drugs are used. Chloroquine and hydroxychloroquine have been used for many years for the treatment and prophylaxis of malaria. They are used to treat autoimmune disorders such as systemic lupus erythematosus and rheumatoid arthritis because of their immunomodulatory effects. Furthermore, chloroquine has been shown to have significant in-vitro effects antiviral against a variety of viruses, including influenza A, human immune deficiency virus (HIV), and more recently, severe acute respiratory syndrome corona virus (SARS-CoV or SARS-CoV-1) [6-8]. Chloroquine and hydroxychloroquine are currently under investigation for the treatment of the COVID-19. Currently, at least 80 trials of chloroquine, hydroxychloroquine, with and without combination with other drugs, are registered worldwide [9].
The mechanism of action is likely related to alteration of endosomal pH required for virus/cell fusion [10] and interference with glycosylation of angiotensin-converting enzyme-2 receptors which are the binding receptors for SARS-CoV-2 [8].
Drug-induced QT prolongation is used as a surrogate indicator for increased risk of drug-associated torsades de pointes (TdP), a potentially lethal polymorphic ventricular tachycardia. The risk of drug-induced TdP is increased in the presence of other risk factors such as female gender, electrolyte disturbances, congenital LQTS, structural heart disease, hepatic failure, renal failure,
and concomitant use of QT-prolonging medications [11]. Life-threatening severe QT prolongation in a patient with prolonged use of hydroxychloroquine has been reported. (2, 3) There is limited data suggest improved outcomes of moderate-severe acute respiratory distress syndrome (ARDS) with adjunctive use of azithromycin [12]. Azithromycin may also lead to QT prolongation and possible TdP [13].
Furthermore, chloroquine and hydroxychloroquine are metabolized by CYP3A4 and using it in combination with antiviral drugs such as ritonavir/lopinavir (both potent CYP3A4 inhibiting drugs and their combination is associated with QT prolongation), or remdesivir (possible QT-prolonging effect is not yet apparent), might result in higher plasma levels and significant QT-prolongation [14].
There are other potential cardiovascular complications of chloroquine, and hydroxychloroquine, including direct myocardial toxicity with cardiomyopathy or exacerbation of underlying cardiomyopathy and altered cardiac conduction e.g., atrioventricular (AV) block, and bundle branch block [11]. The main goal of QTc screening in COVID-19 patients is to identify those at increased risk for TdP, so aggressive measures may be implemented to reduce this risk.
Our patient had a QTc prolongation of 75 ms on day-1 post-initiation of hydroxychloroquine and remained the same for the course of treatment. In recently published studies looking at QT prolongation in patients with SARS-CoV-2 infection treated with hydroxychloroquine/ azithromycin combination, the prolongation of QTc > 500 ms was noted in 17.5-20% of the patients [15-18], change in QTc (ΔQTc) > 60 ms from baseline ECG was observed in 11-25% of the patients [15,17,18], and Tdp was seen in two patients [15,17]. The medication has to be discontinued by 3.5% to 17.5 % of the patients due to QT prolongation[15,17-19]. The combination regimen was associated with more increase in QTc compared to chloroquine/hydroxychloroquine alone in three studies [17-19]. Creatinine and co-administration of amiodarone were predictors of ΔQTc> 60 ms in one study [15]and QTc ≥500 ms was more significant with concomitant use of loop diuretic [17], co-administration of amiodarone [15] and baseline QTc [15,17] see Table 1.
It is unclear if there were patients with congenital LQTS in these studies. It is recommended to stop hydroxychloroquine or other drugs that cause QT prolongation if QTc increased by 60 ms or more. Despite the increase in QTc of > 60 ms in our patient, she did not have any significant arrhythmia. In our patient, steps were taken to minimize the TdP risk by correcting electrolytes and reducing the number of medications that cause QT prolongation.
Conclusion
Chloroquine and hydroxychloroquine and antiviral drugs are promising medications for treating COVID-19 patients. They have the potential of causing QT prolongation and malignant ventricular arrhythmias, among other possible cardiovascular complications. It is essential to reduce this risk by taking care of all other reversible contributing factors for prolongation. Fortunately, the therapy duration in this setting is short, and it is likely with close cautious monitoring to avoid these side effects. More data about using chloroquine/hydroxychloroquine and other drugs used in treating COVID-19 patients in patients with LQTS is needed. Hopefully, this may be obtained from the ongoing registries and randomized controlled studies. The use of these medications in COVI-19 alone or in combination with other drugs should be individualized based on the potential benefits compared to the possible side effects.
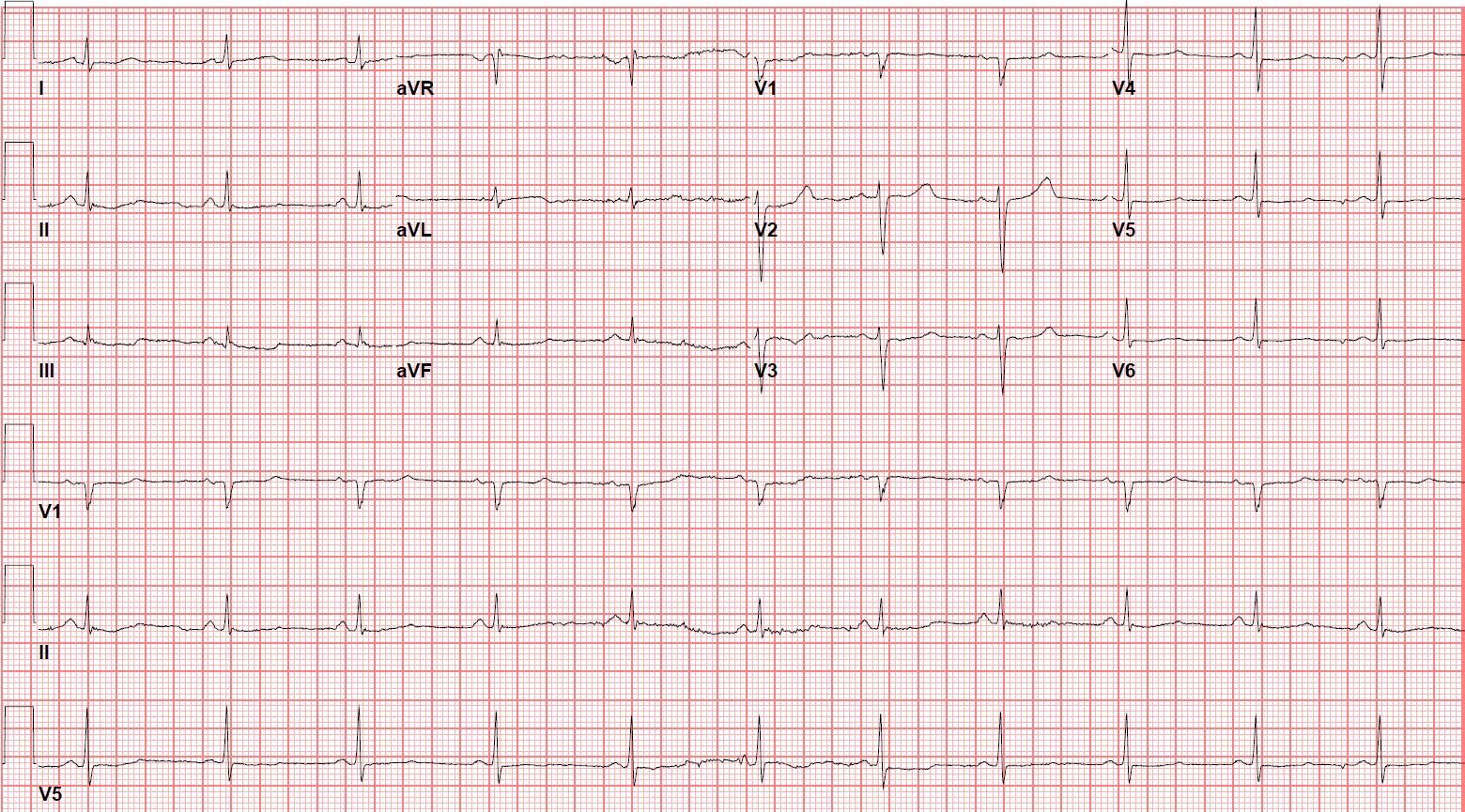
Figure 1a: ECG on admission showing sinus rhythm 66 beats per minute and QTc 454 ms.
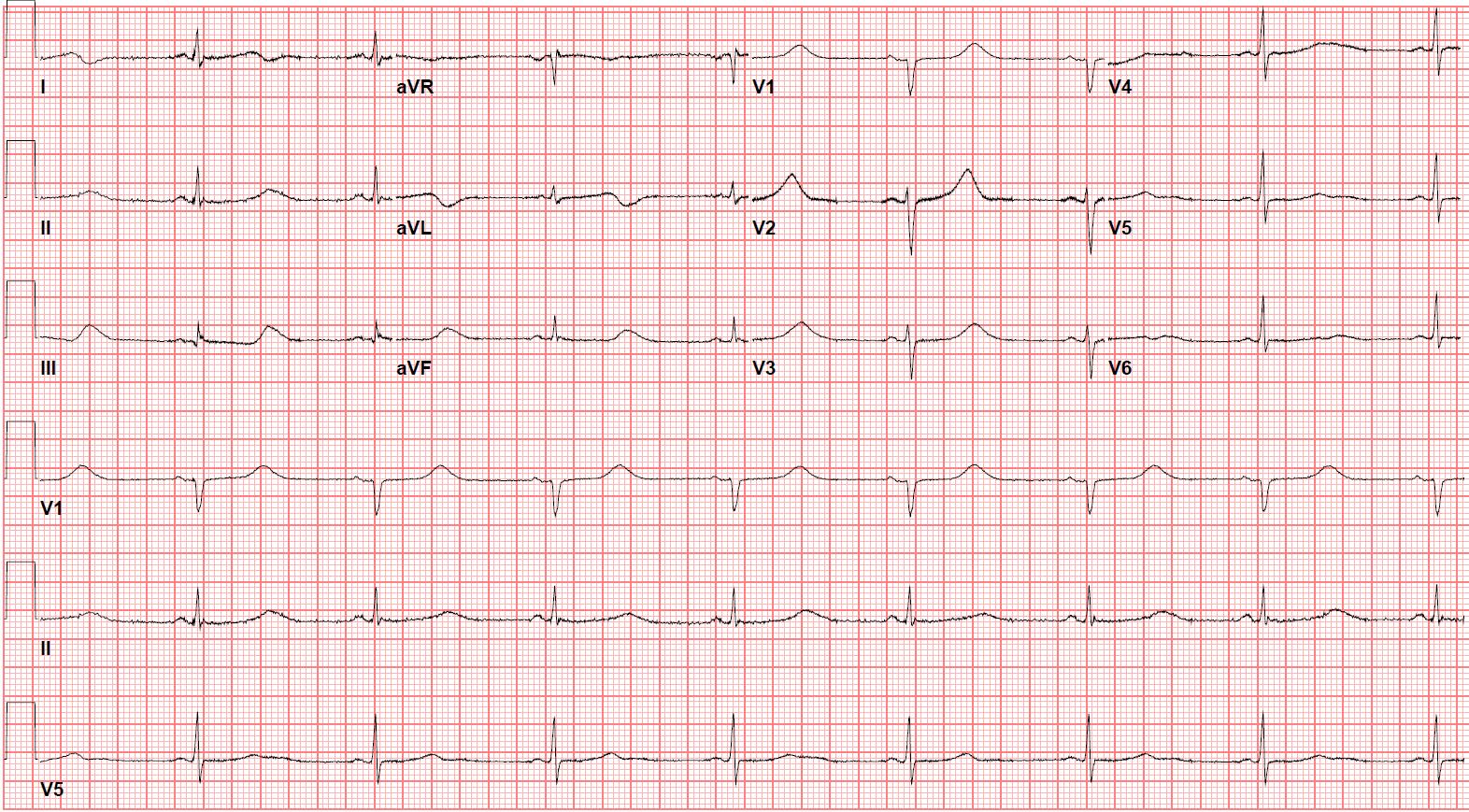
Figure 1b: ECG day one post initiation of hydroxychloroquine showing sinus rhythm with QTc 529 ms
|
- |
(251 patients) [15] |
(117 patients) [16] |
(90 patients) [17] |
(40 patients) [18] |
(201 patients) [19] |
|
QTc > 500 ms |
23% |
17.9% |
20% (19% in HCQ vs. 21% HCQ/AZ) |
17.5 % |
The maximum QTc 470.4 ± 45.0 ms vs. 453.3 ± 37.0 ms |
|
QTc change |
> 60 ms in 20% |
Averageincrease 33.9 ± 26.8ms |
>60 ms in 11% (3% VS 13%) |
>60 ms in 25% |
- |
|
Significant difference in QTc prolongation between CQ or HCQ alone and CQ or HCQ/AZT combination |
Only combination |
No |
Yes |
Yes (5% vs 33% QTc > 500 ms) |
Yes |
|
Predictor of QTc> 500 ms |
Acute renal failure |
- |
Concomitant loop diuretic and a baseline QTc of > 450 ms |
- |
- |
|
Predictors of ΔQTc> 60 ms |
Baseline Creatinine and co-administration of Amiodarone |
- |
- |
- |
- |
|
Discontinuation of treatment |
8 patients (3.2%) |
- |
10 patients (11%) |
7 patients (17.5%) due abnormalECG and10 patients (25%) due acute renal failure |
7 patients (3.5%) |
|
Torsade de pointes (TdP) |
1 patient |
None |
1 patient |
None |
None |
Table 1: Summary of recently published studies comparing QTc prolongation with chloroquine (CQ)/ hydroxychloroquine(HCQ) alone or, combined with azithromycin (AZT) in patients with COVID-19.
Citation: Al-Ghamdi B, Echahidi MN, Edathodu J, Alrajhi A (2020) Management of Corona Virus Disease 2019 in a Patient with a Congenital Long-QT Syndrome: A Case Report. Ann Med & Surg Case Rep: AMSCR-100066