Annals of Medical & Surgical Case Reports
ISSN 2652-4414
Volume 02; Issue 04
Research Article
The Historical/Evolutionary Cause and Possible Treatment of Pandemic Covid-19 (Sars-Cov-2, 2019-Coronavirus)
Niknamian S1* and Zaminpira S2
1Department of Military Medicine,American Military University and Liberty University, USA
2Department of Phycology and Psychiatry,American Beirut University, Beirut, Lebanon
*Corresponding author: Sorush Niknamian, Department of Military Medicine, American Military University and Liberty University, USA.
Citation: Niknamian S and Zaminpira S (2020) The Historical/Evolutionary Cause and Possible Treatment of Pandemic Covid-19 (Sars-Cov-2, 2019-Coronavirus). Ann Med &Surg Case Rep: AMSCR-100050
Received date: 11 April, 2020; Accepted date: 20 April, 2020; Published date: 27 April, 2020
Abstract
Background: A virus is a small infectious agent that replicates only inside the living cells of an organism. Viruses can infect all types of life forms, from animals and plants to microorganisms, including bacteria and archaea. In evolution, viruses are an important means of horizontal gene transfer, which increases genetic diversity in a way analogous to sexual reproduction.Influenza (Including (COVID-19), is an infectious disease caused by an influenza virus. Some viruses especially smallpox, throughout history, has killed between 300-500 million people in its 12,000-year existence.As modern humans increased in numbers, new infectious diseases emerged, including SARS-CoV-2. We have two groups of virus, RNA and DNA viruses. The most brutal viruses are RNA ones like COVID-19 (Sars-CoV-2).
Introduction:Coronaviruses are a group of viruses that cause diseases in mammals and birds. In humans, coronaviruses cause respiratory tract infections that are typically mild, such as some cases of the common cold (among other possible causes, predominantly rhinoviruses), though rarer forms can be lethal, such as SARS, MERS, and COVID-19. Symptoms vary in other species: in chickens, they cause an upper respiratory tract disease, while in cows and pigs they cause diarrhea. Coronaviruses constitute the subfamily Orthocoronavirinae, The genome size, coronaviruses ranges from approximately 27 to 34 kilobases, the largest among known RNA viruses.
Discussions and Results:We have researched from the first virus in the planet to the last mutated version which is SARS-COV-2. We have collected many informative data in tables and figures to reach the main cause of 2019-Coronavirus and calculated the probability and estimated deaths in the current time. We have discussed about the possible treatment and prevention of the virus and did algebraic calculations on the epidemiology, the size and even the future of this pandemic. The only era which any virus had not been epidemic, were through world war 2, were the German scientists had found the way to fight any viral infections which is very important and can help scientists to reach the main treatment of the new 2019-Coronavirus. We have sorted the deadly and non-deadly coronaviruses and explained how this epidemic had begun through Evolutionary Medicine (EM). The result of the article is that 16% of the whole population in the world has been contaminated which is 1248000000 of 7.8 billion people world-wide. SARS-CoV-2 is an RNA Virus. its nucleic acid is single-stranded RNA (ssRNA). The polarity of this virus is positive-sense ((+) ssRNA). Positive-sense viral RNA is similar to mRNA and thus can be immediately translated by the host cell. Recombination in RNA viruses appears to be an adaptation for coping with genome damage. Recombination can occur infrequently between animal viruses of the same species but of divergent lineages. The resulting recombinant viruses may sometimes cause an outbreak of infection in humans. RNA viruses have very high mutation rates This is one reason why it is difficult to make effective vaccines to prevent diseases caused by RNA viruses. The resulting recombinant viruses causes an outbreak of infection in humans.
Conclusion:In conclusion, the mutation of the SARS-CoV and influenza viruses through Drift and Reassortment is the main cause of SARS-CoV-2 through natural selection, Lamarckian Evolution and coevolution which caused this RNA virus so powerful, unpredicted and different in the genome size and nations worldwide. The first Pandemic of Influenza was first detected in 1732 and this virus evolved through natural selection till 2019 which caused the worldwide pandemic of SARS-CoV-2. Based on many studies, inhalation of Ozone plus Sulfur Dioxide, increasing the amounts of L-Glutathione (Which is low in children and older adults and this is the main reason why older adults and children die from this disease.) plus Viral Phage Therapy (VPT) which we discussed fully in this article can be the possible prime treatment of SARS-CoV-2 infection. The seasonal temperature cannot be useful in controlling/reducing the pandemic of this virus since the natural selection, Lamarckian Evolution and high mutation of the virus helps its survival. No antiviral drugs will be useful against SARS-CoV-2 because of high rate of mutation and primarily adaptation of the virus to the drugs and even the environmental Temperature.
Keywords: COVID-19 (SARS-CoV-2);Drift; Fibonacci Series; Influenza; Lamarckian Evolution; L-Glutathione; Natural Selection;Ozone; Positive-Sense ((+) ssRNA Sulfur Dioxide; Reassortment; Pandemic); Viral Phage Therapy
Introduction
Brief History of Viruses
Prehistory: The world, new infectious diseases emerged, including those caused by viruses[1]. Earlier, humans lived in small, isolated communities, and most epidemic diseases did not exist[2,3]. Smallpox, which is the most lethal and devastating viral infection in history, first emerged among agricultural communities in India about 11,000 years ago[4]. The virus, which only infected humans, probably descended from the poxviruses of rodents [5]. Humans probably came into contact with these rodents, and some people became infected by the viruses they carried. When viruses cross this so-called "species barrier", their effects can be severe, [6] and humans may have had little natural resistance. Contemporary humans lived in small communities, and those who succumbed to infection either died or developed immunity.
This acquired immunity is only passed down to offspring temporarily, by antibodies in breast milk and other antibodies that cross the placenta from the mother's blood to the unborn child's. Therefore, sporadic outbreaks probably occurred in each generation. In about 9000 BC, when many people began to settle on the fertile flood plains of the River Nile, the population became dense enough for the virus to maintain a constant presence because of the high concentration of susceptible people [7]. Other epidemics of viral diseases that depend on large concentrations of people, such as mumps, rubella and polio, also first occurred at this time [8]. The Neolithic age, which began in the Middle East in about 9500 BC, was a time when humans became farmers [9].
This agricultural revolution embraced the development of monoculture and presented an opportunity for the rapid spread of several species of plant viruses [10]. The divergence and spread of sobemoviruses-southern bean mosaic virus-date from this time[11]. The spread of the potyviruses of potatoes, and other fruits and vegetables, began about 6,600 years ago. [10] About 10,000 years ago the humans who inhabited the lands around the Mediterranean basin began to domesticate wild animals. Pigs, cattle, goats, sheep, horses, camels, cats and dogs were all kept and bred in captivity[12]. These animals would have brought their viruses with them[13]. The transmission of viruses from animals to humans can occur, but such zoonotic infections are rare and subsequent human-to-human transmission of animal viruses is even rarer, although there are notable exceptions such as influenza. Most viruses are species-specific and would have posed no threat to humans[14].
The rare epidemics of viral diseases originating in animals would have been short-lived because the viruses were not fully adapted to humans [15] and the human populations were too small to maintain the chains of infection[16]. Other, more ancient, viruses have been less of a threat. Herpes viruses first infected the ancestors of modern humans over 80 million years ago[17].Humans have developed a tolerance to these viruses, and most are infected with at least one species[18]. Records of these milder virus infections are rare, but it is likely that early hominids suffered from colds, influenza and diarrhoea caused by viruses just as humans do today. More recently evolved viruses cause epidemics and pandemics[17]. The influenza virus is one that seems to have crossed the species barrier from ducks and water fowl to pigs and from there to humans. It is possible that a fatal plague in the Middle East at the time of the late 18th Dynasty was associated with this transmission at Amarna [19-20].
Influenza:Influenza, commonly known as the flu, is an infectious disease caused by an influenza virus [21-25]. Symptoms can be mild to severe [25]. The most common symptoms include: high fever, runny nose, sore throat, muscle and joint pain, headache, coughing, and feeling tired just like Sars-CoV-2[21]. These symptoms typically begin two days after exposure to the virus and most last less than a week [21]. The cough, however, may last for more than two weeks [21]. In children, there may be diarrhea and vomiting, but these are not common in adults [26]. Diarrhea and vomiting occur more commonly in gastroenteritis, which is an unrelated disease and sometimes inaccurately referred to as stomach flu or the 24-hour flu[26]. Complications of influenza may include viral pneumonia, secondary bacterial pneumonia, sinus infections, and worsening of previous health problems such as asthma or heart failure [22,25]. Three of the four types of influenza viruses affect humans: Type A, Type B, and Type C[22,27]. Type D has not been known to infect humans, but is believed to have the potential to do so [27,28]. Usually, the virus is spread through the air from coughs or sneezes [21](Table 1-7). This is believed to occur mostly over relatively short distances [29]. It can also be spread by touching surfaces contaminated by the virus and then touching the eyes, nose, or mouth [25,29,30-35]. A person may be infectious to others both before and during the time they are showing symptoms [25,36-40]. The infection may be confirmed by testing the throat, sputum, or nose for the virus [45-50]. A number of rapid tests are available; however, people may still have the infection even if the results are negative [22]. A type of polymerase chain reaction that detects the virus's RNA is more accurate [22](Figure 1-3).
List of epidemics of Plagues
Coronaviruses are a group of viruses that cause diseases in mammals and birds. In humans, coronaviruses cause respiratory tract infections that are typically mild, such as some cases of the common cold (among other possible causes, predominantly rhinoviruses), though rarer forms can be lethal, such as SARS, MERS, and COVID-19. Symptoms vary in other species: in chickens, they cause an upper respiratory tract disease, while in cows and pigs they cause diarrhea. Coronaviruses constitute the subfamily Orthocoronavirinae, in the family Coronaviridae, order Nidovirales, and realm Ribavirin [31,32]. They are enveloped viruses with a positive-sense single-stranded RNA genome and a nucleocapsid of helical symmetry. The genome size of coronaviruses ranges from approximately 27 to 34 kilobases, the largest among known RNA viruses[33].
Evolution
The most recent common ancestor (MRCA) of all coronaviruses has been placed at around 8000 BCE [34]. The MRCAs of the Alpha-coronavirus line has been placed at about 2400 BCE, the Beta-coronavirus line at 3300 BCE, the Gamma-coronavirus line at 2800 BCE, and the Delta-coronavirus line at about 3000 BCE. It appears that bats and birds, as warm-blooded flying vertebrates, are ideal hosts for the coronavirus gene source (with bats for Alpha-coronavirus and Beta coronavirus, and birds for Gamma-coronavirus and Delta-coronavirus) to fuel coronavirus evolution and dissemination [35]. Bovine coronavirus and canine respiratory coronaviruses diverged from a common ancestor in 1951 [36]. Bovine coronavirus and human coronavirus OC43 diverged around the 1890s. Bovine coronavirus diverged from the equine coronavirus species at the end of the 18th century [37]. The MRCA of human coronavirus OC43 has been dated to the 1950s [38]. MERS-CoV, although related to several bat coronavirus species, appears to have diverged from these several centuries ago [39]. The human coronavirus NL63 and a bat coronavirus shared an MRCA 563-822 years ago [40]. The most closely related bat coronavirus and SARS-CoV diverged in 1986 [41]. A path of evolution of the SARS virus and keen relationship with bats have been proposed. The coronaviruses have been coevolved with bats for a long time and the ancestors of SARS-CoV first infected the species of the genus Hipposideridae, subsequently spread to species of the Rhinolophidae and then to civets, and finally to humans[42-43]. Alpaca coronavirus and human coronavirus 229E diverged before 1960 [44].
Discussion
By mentioning all the facts, evolution and all the similarities from the viruses, we observe that all kinds of Flu including COVID-19 which have been caused many deaths through epidemic and pandemic of these giant but very small objects, all the viruses from the first one before the rise of the human in evolution, had been existed. Through my deep research, the first virus was or blue-green algae, pre-date viruses and are one of the oldest forms of life on Earth(Figure 4).
Results
The researchers developed algorithms to compare the protein shapes of 3,460 viruses and 1,620 cells. They found that 442 protein folds were shared between cells and viruses, but 66 folds were unique to viruses. To make sense of the data, the team arranged the protein folds into a tree that grew a new 'branch' every time a new type of protein fold evolved. Wherever possible, the team used fossil evidence to put an approximate date on the budding of specific branches. For example, one particular protein fold was first seen in cyanobacteria (blue-green algae), and later appeared in all its descendants. By comparing when cyanobacteria first appeared in the fossil record (2.1 billion years ago) to when its offspring later emerged, they could establish this particular fold appeared around 2 billion years ago. According to Caetano-Anolles’s microbial family tree, viruses are ancient, however; they were not the first form of life. In fact, his family tree suggests viruses and bacteria share a common ancestor - a fully functioning, self-replicating cell that lived around 3.4 billion years ago, shortly after life first emerged on the planet. From this cell, bacteria have evolved in the direction of increasing complexity, while viruses have gradually shed genes they found they didn’t need, until they could no longer even reproduce on their own. A key step in the virus evolutionary journey seems to have come about around 1.5 billion years ago and that’s the age at which the 66 virus-specific protein folds came on the scene. These changes are to proteins in the virus’ outer coat-the machinery viruses use to break into host cells. For Charleston, the remarkable point about the study is how many proteins today’s bacteria and viruses have in common.Viral evolution, Primordial cellular origins and late adaptation to parasitism, Mob Genet Elements(Figure 5).
Drift
New viruses can also emerge by drift. Drift can refer to genetic drift or antigenic drift. [45] Mutation and selection for the most advantageous variation of the virus takes place during this form of evolution. Natural Selection and Lamarckian Evolution both caused the evolution of several viruses like Coronaviruses fast enough[45]. Antigenic mutants can evolve quickly due to the high mutation rate in viruses. The cause of the antigenic drift lies in the mechanisms of RNA synthesis itself. Mutations arise very easily simply due to the error prone RNA polymerase and its lack of proofreading mechanisms. These mutations lead to subtle changes in the HA and NA genes which completely changes the infectious capabilities of the virus. These changes allow for almost endless possibilities for new viral strains to arise [46,47] and it is the antigenic drift of the HA and NA genes that allow for the virus to infect humans that receive vaccines for other strains of the virus[48]. This evolution occurs under the pressure of antibodies or immune system responses. Seven strains of human coronaviruses are known and four of them produce the generally mild symptoms of the common cold: 1) Human coronavirus OC43 (HCoV-OC43), 2) Human coronavirus HKU1, 3) Human coronavirus NL63 (HCoV-NL63, New Haven coronavirus), 4) Human coronavirus 229E (HCoV-229E) and three coronaviruses that are potentially fatal: 5) Middle East respiratory syndrome-related coronavirus (MERS-CoV), previously known as novel coronavirus 2012 and HCoV-EMC, 6) Severe acute respiratory syndrome coronavirus (SARS-CoV or "SARS-classic"), 7) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), previously known as 2019-nCoV or "novel coronavirus 2019"[45].
Reassortment
Reassortment, also known as antigenic shift, allows new viruses to evolve under both natural conditions and in artificial cultures[45].Reassortment occurs in a similar way as chromosome crossover events, as two different viral strains may come in contact and transfer some of their genetic information. This crossing-over event creates a mixture of the two viral strains, which may replicate as one hybrid virus that expresses traits from both original viruses[46]. The mechanism of the evolutionary force of antigenic shift allows influenza viruses to exchange genes with strains that infect different species. Under this mechanism, a human influenza virus could exchange genes with an avian strain, and that is how pandemic strains arise. There have been three occurrences of pandemics caused by antigenic shift since 1900, and it has happened again today as we know as SARS-CoV-2[47].In fact, the 1957 evolution of the H2N2 virus is thought to be a result of Reassortment[45]. In this case, human H1N1 strains and avian influenza A genes were mixed [45]. Infecting tissue cultures can demonstrate how pathogenic qualities can evolve for a particular species even though the reasserted virus may be nonpathogenic for another species [45]. A prime example of evolution under natural conditions is the Reassortment of two avian influenza strains that were discovered in dead seals back in 1979 [45].
Based on research by an Inhalation of ozone and sulfur dioxide inhibited influenza virus growth in the nose of mice. Ozone inhalation caused the more pronounced inhibition of influenza virus growth: 0.6 ppm ozone for 3 hours’ post-virus exposure almost completely inhibited influenza virus growth in the nose, whereas sulfur dioxide (6 ppm for 7 days) causes only partial inhibition of influenza growth in the nose. Neither gas altered the propagation of influenza virus in the lungs of mice. Vesicular stomatitis virus (VSV) growth was either unaffected by exposure to ozone (0.9 ppm for 3 hours) or, when ozone exposure preceded VSV exposure, the virus may have grown to slightly higher titer. The inhibitory effect of ozone and sulfur dioxide on influenza virus growth in nasal epithelium suggests a competitive interaction between the chemical inhalant, the virus, and host tissues, with net consequences for the pathogenesis of this disease. If the effects of these inhalants are to be properly interpreted, they should be determined for all major regions of virus growth and inhalant deposition. Therefore; inhalation of Ozone plus Sulfur Dioxide may inhibit the influenza virus growth in humans as well.
Another research indicates that Influenza virus was rendered noninfectious for mice by in vitro exposure to relatively high concentrations of sulfur and nitrogen mustards. Concentrations of the mustards that abolished infectivity did not reduce the ability of the virus to agglutinate erythrocytes. The parenteral administration of nitrogen mustard had no effect on the course of infection with influenza virus in mice.
There are several researches on the compound L-Glutathione which contains high sulfur amounts and it is normally produced by liver but the amounts vary in ages and sexes in its compound and inhibits viral infection. These researches has done in in Vivo and in Vitro with controlled groups double blind and received totally positive results and the treatment of the patients with several most dangerous viral diseases including Coronaviruses.
Sulfur and Viruses
Scientists have known about viruses that attack bacteria since the early twentieth century. In 1917, Felix d’Herelle, a French-Canadian microbiologist, and his colleagues successfully isolated phages that kill bacteria like E. coli, salmonella, and dysentery, and doctors used his so-called phage therapies to treat disease. But phage research lost steam in the 1950s in the face of penicillin and other powerful new antibiotics.Bacteria are living micro-organism which can be affected by viruses. The adaptation of bacteria to fight viruses are higher than human living cells. Therefore; this is the most important research on Phages against viruses which should be considered by scientists to fight against all viruses including 2019-Coronavirus. Paula Dalcin Martins and his colleagues reached positive results using Sulfur against many viruses. Basically If two viruses share a good number of genes, they are probably related like MERS, SARS and 2019-Coronavirus.The fewer genes they share, the more divergent their evolutionary paths. The most impressive article about sulfur against viruses has been published by Matthew B Sullivan which indicated Sulfur can kill and terminate any known viruses in eukaryotic host cells. Based on Sullivanstatements and articles, Sulfur and Phage Therapies can be one of the main treatment of viral diseases through coevolution hypothesis (coevolution occurs when two or more species reciprocally affect each other's evolution through the process of natural selection like viruses and mammals.) including SARS-CoV-2(Figure 7-10).
In Taxonomy, the family Coronaviridae is organized in 2 sub-families, 5 genera, 23 sub-genera and about 40 species: Coronaviridae - Orthocoronavirinae -Letovirinae-Alphaletovirus-Milecovirus and then Microhylaletovirus[3].
MERS-CoV and Severe acute respiratory syndrome (SARS) are betacoronavirus derived from bats [2].Between November 2002 and July 2003, an outbreak of SARS in southern China caused an eventual 8,098 cases, resulting in 774 deaths reported in 17 countries (9.6% fatality rate), with the majority of cases in mainland China and Hong Kong. No cases of SARS have been reported worldwide since 2004. In late 2017[1-3]. Chinese scientists traced the virus through the intermediary of civets to cave-dwelling horseshoe bats in Yunnan province[220]. Therefore; through the lines above, SARS and COVID-19 outbreak was first observed in China.The coronaviruses HCoV-229E, -NL63, -OC43, and -HKU1 continually circulate in the human population and cause respiratory infections in adults and children world-wide [221].
In the new Coronavirus, Genetic recombination which is the process by a strand of DNA that is broken and then joined to the end of a different DNA molecule. This can occur when viruses infect cells simultaneously and studies of viral evolution have shown that recombination has been rampant in the species studied. [230]. Recombination is common to both RNA and DNA viruses [108,220]. SARS-CoV-2 has undergone genetic change by several mechanisms. These include a antigenic drift process where individual bases in the RNA mutate to other bases. the mutations of this virus is silent means it does not change the protein that the gene encodes. And confers evolutionary advantages such as resistance to antiviral drugs [210-220]. Therefore; we cannot mention there would be any possible antiviral drugs to resist this kind of virus even in the future.
SARS-CoV-2 is an RNA Virus. its nucleic acid is single-stranded RNA (ssRNA). The polarity of this virus is positive-sense ((+) ssRNA). Positive-sense viral RNA is similar to mRNA and thus can be immediately translated by the host cell. Recombination in RNA viruses appears to be an adaptation for coping with genome damage. Recombination can occur infrequently between animal viruses of the same species but of divergent lineages. The resulting recombinant viruses may sometimes cause an outbreak of infection in humans. RNA viruses have very high mutation rates. This is one reason why it is difficult to make effective vaccines to prevent diseases caused by RNA viruses[215,216].
Mathematical Calculation of SARS-CoV-2 and Future Concerns: The Factorial formula is:
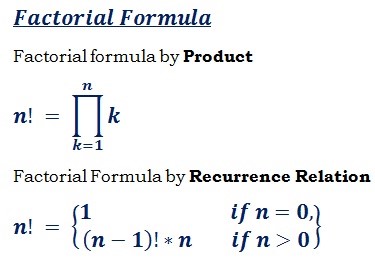
The size of SARS-CoV-2 is approximately 0.3 microns[237]. With conversion of Micrometer into nanometer, 0.3 will be 300 nm in size. In page 21, we have described the Antigenic Drift of viruses especially SARS-CoV-2. The mathematical calculation of how many types of this virus exists is: 3 × 4, since we have 4 types of mild coronavirus and 3 types of deadly with severe symptoms. So the numbers of different coronaviruses will be 3 × 4=12. Therefore; based on each type of virus is different from another one, we use Factorial to measure all types of coronavirus which exists on earth till now without the concern of the mutation in vivo of the virus. It is 12!=479001600. Overall, the number of different SARS-CoV-2 is about 479001600. Each of these kinds of viruses when get inside their hosts also mutates. Therefore; Each of these coronaviruses get inside their host will have different symptoms from mild into severe based on the host’s immune system response and other factors. Hence; all these SARS-CoV-2, will mutate and possible severe symptoms will occur based on the Reassortment of the virus which have been described in page 22.The probability of each person in the world who may contaminate with this virus is 7.8 Billion/479001600 equals 16.2838704505.
This is the probability from the first time outbreak of the virus means approximately 16% of the population infected by the virus. So the near future of death or infected people from SARS-CoV-2 will be 1248000000. Based on high rates of mutation of 2019-Corovirus, each carrier of this deadly virus may/will contaminate another living human. Therefore; all people will carry this virus in the near future. We do not know the time range of this highly pandemic, but the lesson that we have learnt from the history is that the only way to fight the virus will not producenew vaccines, since the adaptation and the mutation of the virus is higher than any knownones on earth. The only answer to end the pandemic, is the adaptation of human race to SARS-CoV-2 through Lamarckian Evolution. This infectious disease with 7.8 billion people living on earth is like playing with dices and the people contamination is random in mathematical Theorems. Some may adapt to the virus, some will die and the rest will be the carrier, therefore; the graph we predict is like The bean machine, a device invented by Francis Galton shown below(Figure 11):
According to the central limit theorem (more specifically, the de Moivre-Laplace theorem), the binomial distribution approximates the normal distribution provided that the number of rows and the number of balls are both large. Varying the rows will result in different standard deviations or widths of the bell-shaped curve or the normal distribution in the bins [219].
2019-Corovirus Relation to Fibonacci Sequence: The Fibonacci numbers, commonly denoted Fn, is a function called the Fibonacci sequence, means each number is the sum of the two preceding numbers, starting from 0 and 1:
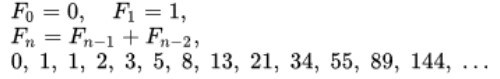
And the sequence is: We have collected the infected people with COVID-19. If we suppose each infected individual infects one other individual each day and at the end of day-two recovers fully or dies (we are interested in the number of people who are actively infected and alive). So the time stamps are the beginning of day-one, at the end of day-one and at the end of day-two. According to our model, an infected individual can infect one other person at the end of day-one and at the end of day-two. If we go on through time (days), the table below shows the result of infected people (Figure 12-14).
Therefore; the formula of infected people per day is: 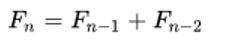 ???????(for n > 2), which is the formula of Fibonacci series.
???????(for n > 2), which is the formula of Fibonacci series.
Viruses Related to COVID-2019
There are some viruses that causes respiratory infection. Enteroviruses, Poliovirus, coxsackie, echovirus and Human orthopneumovirus. Hence; none of them has been related to 2019-Coronavirus. Since their Signs and symptoms, Structure, Genome, Evolution, Taxonomy and Transmission are not similar to the new COVID-2019. The only virus that has been correlated with COVID-2019 is Rhinovirus. The rhinovirus is the most common viral infectious agent in humans and is the predominant cause of the common cold and flu. Rhinovirus infection proliferates in temperatures of 33-35 °C (91-95 °F), the temperatures found in the nose. The three species of rhinovirus (A, B, and C) include around 160 recognized types of human rhinovirus that differ according to their surface proteins (serotypes) They are lytic in nature and are among the smallest viruses, with diameters of about 30 nanometers. By comparison, other viruses, such as smallpox and vaccinia, are around ten times larger at about 300 nanometers (the same diameter as COVID-2019 which is 300 nanometers in size)[220].
There are two modes of transmission: via aerosols of respiratory droplets and from fomites (contaminated surfaces), including direct person-to-person contact. Rhinoviruses are spread worldwide and are the primary cause of the common cold. Symptoms include sore throat, runny nose, nasal congestion, sneezing and cough. Those most affected by rhinoviruses are infants, the elderly, and immune compromised people (Most elderly and Children). Human rhinoviruses preferentially grow at 32 °C (89 °F), notably colder than the average human body temperature of 37 °C (98 °F); hence the virus's tendency to infect the upper respiratory tract, where respiratory airflow is in continual contact with the (colder) extrasomatic environment [221].
Rhinoviruses have single-stranded positive sense RNA genomes of between 7200 and 8500 nt in length. At the 5' end of the genome is a virus-encoded protein, and like mammalian mRNA, there is a 3' poly-A tail. Structural proteins are encoded in the 5' region of the genome and nonstructural at the 3' end. This is the same for all picornaviruses. The viral particles themselves are not enveloped and are icosahedral in structure. The viral proteins are translated as a single, long polypeptide, which is cleaved into the structural and nonstructural viral proteins [212]. Human rhinoviruses are composed of a capsid that contains four viral proteins, VP1, VP2, VP3 and VP4.VP1, VP2, and VP3 form the major part of the protein capsid. The much smaller VP4 protein has a more extended structure, and lies at the interface between the capsid and the RNA genome. There are 60 copies of each of these proteins assembled as an icosahedron. Antibodies are a major defense against infection with the epitopes lying on the exterior regions of VP1-VP3. Human rhinovirus is most contagious during the autumn and winter months. The virus can remain activated for up to 3 hours outside of a human host. Once the virus is contracted, a person is most contagious within the first 3 days. Preventive measures such as regular vigorous hand washing with soap and water may aid in avoiding infection. Avoiding touching the mouth, eyes and nose, the most common entry points for rhinovirus, may also aid in prevention. Droplet precautions, which take the form of a surgical mask and gloves, is the method used in major hospitals.[243] [214,215].As mentioned, through our analysis, the most related virus to COVID-2019, is Rhinovirus which has very similar in Structure, Genome, Size and symptoms of the SARS-CoV-2. There has not been an effective anti-viral vaccination of this virus either.
There is a high possibility of mutation in this virus into 2019-Coronavirus. In 2018, a new series of anti-rhinoviral compounds were reported by researchers at Imperial College London and colleagues at the University of York and the Pirbright Institute. These molecules target human N-myristoyltransferase, an enzyme in the host cell which picornavirus requires in order to assemble its viral capsid, and thus generate an infectious virion. The lead compound in this series, IMP-1088, very potently inhibited host myristoylation of viral capsid protein and prevented infectious virus formation, rescuing the viability of cells in culture which had been exposed to a variety of rhinovirus serotypes, or to related picornaviruses including poliovirus and foot-and-mouth-disease virus. Because these compounds target a host factor, they are broadly active against all serotypes, and it is thought to be unlikely that they can be overcome by resistance mutations in the virus.
The main Cause of COVID-19 Pandemic
Through the lines and statements in this article, we hypothesize that the prime cause of SARS-CoV-2 is:
The Possible Treatment of COVID-19 (SARS-CoV-2)
Based on our research/Review of many major researches, there has not been any antiviral vaccines against these viruses and no antiviral drugs will be useful against COVID-19 since the adaptation and high mutation of this virus will not let these drugs be effective. The prime possible treatment Inhalation of ozone and sulfur dioxide, increasing L-Glutathione Plus Viral Phage Therapy (VFT) is the possible prime treatment of this disease. Finding the right Phage Therapy is very hard in any viral infections since this therapy was mainly used against bacterial infections. However; through our research and findings we have concluded that substantial dose-dependent inhibition by T4 phage of adsorption of HAdV to both A549 and HEK293 cell lines in vitro LPS was without effect. Moreover, T4 phage protected A549 cells from anHAdV-induced cytopathic effect. These data suggest that T4 phage could be considered as a potential novel antiviral agent. The capacity of the phage to inhibit HAdV infection at the stage of viral replication suggests that phage could also interfere with viruses using cellular receptors other than those used by HAdV. Notably, the anti-viral effect of phage was measured as the decrease of the end-point HAdV infectious titer. Further in vitro studies were carried out on the effects of T4 and the staphylococcal phage A5/80 on HAdV DNA synthesis (real-time PCR) and the expression of its early and late genes (measured at the level of mRNA synthesis) in an A549 cell culture. Continuous incubation of adenovirus-infected cells with T4 phage significantly reduced the level of adenoviral DNA synthesis (this effect was not observed with the staphylococcal phage). Incubation with a high titer of T4 phage was required for inhibition of HAdV early gene expression. Late adenoviral gene expression was reduced by preincubation (application of phage prior to HAdV infection) and Coincubation with both phages when a low HAdV titer was used. In contrast, when a high HAdV titer was applied, both incubation and preincubation with T4 phage were inhibitory whilst a staphylococcal phage was inhibitory only when continuous incubation with cells was applied. Those results suggest that the inhibitory effect of phage on HAdV infection may differ and may be partially explained by their influence on the expression of early and late adenoviral genes. When the effect of both phages on the expression of genes involved in antimicrobial immunity by the A549 cell line from human lung was studied, the most striking phenomenon was marked (>10-fold) enhancement of a gene coding for interleukin-2 (Il-2) by a staphylococcal phage (Borysowski et al., 2018). This effect is of particular interest in the context of the well-known role of NK cells in the immune response to viruses and the ability of Il-2 to induce those cells - even in ultra-low doses. Interestingly, a resident NK cell population is present in the human lung and may provide early and important control of viral infection (Cooper et al., 2018). Moreover, NK cells also exhibit activity against a variety of bacteria, e.g., by secretion of the soluble molecules perforin and granulysin. Mice infected with Shigella and lacking B and T cells but with normal NK cells have higher survival rates and lower bacterial titers than mice which lack all three cell types. This suggests that phage-induced Il-2 dependent activation of NK-mediated antimicrobial activity may contribute to beneficial effects of PT, especially during prolonged phage administration cumulative median duration of successful phage therapy is 43 days. NK cells could be a promising agent in antimicrobial immunotherapy, as data strongly suggest that these cells are active against not only viral but also bacterial and fungal pathogens.
Acknowledgement
We would like to thank Senator Mark R Warner, Senator Tim Kaine and Representative Ben Cline for their inspirational help and dedication their time and help to the United States of America. Also, we would like to thank Mrs. Fallon Morell the President of Weston A Price Foundation (WAPF), Professor Stephanie Seneff (senior research scientist at the Computer Science and Artificial Intelligence Laboratory (CSAIL), Massachusetts Institute of Technology), Professor Thomas N Seyfried (Morrissey College of Arts and Sciences) and Professor Dominic D’Agostino (Research Scientist, Inst. Human and Machine Cognition, Professor, College of Medicine Molecular Pharmacology & Physiology, Morsani College of Medicine, Molecular Pharmacology & Physiology for their motivational help in my beginning of our first research till now.
Conclusion
In this Research/Review articles, we have gone through all the cases and epidemic of all the fatal diseases from the first time mentioned in history including Flu/Influenza pandemicuntil 2020. Based on our research, the only period of time when there was no outbreak of influenza, RNA viruses or infectious diseases were during World War 2, where German scientists controlled the epidemic of any virus infectionswhich should be noted by the scientists and researchers. The mutation of the SARS-CoV and Influenza viruses is the main cause of SARS-CoV-2 through natural selection and Lamarckian Evolution Both which caused this RNA virus so powerful and different in the nations worldwide. The first Pandemic of Influenza was first detected in 1732 and this virus evolved through natural selection till 2019 which caused the worldwide pandemic of SARS-CoV-2. Based on many studies, inhalation of Ozone plus Sulfur Dioxide, increasing the amounts of L-Glutathione (Which is low in children and older adults) plus Viral Phage Therapy (VPT)can be the prime treatment of SARS-CoV-2 infection. The seasonal temperature cannot be useful in controlling or reducing the pandemic of this virus since the temperature rises gradually and the natural selection, Lamarckian Evolution and high mutation rate of the virus helps its survival. In our mathematical calculation, the number of infected people is/will be 1248000000 which should not be ignored by the scientists. We also introduced the Fibonacci Model in calculating the infected/will be infected people. Through our research we have found that no antiviral drugs will be useful against SARS-CoV-2 because of high rate of mutation and adaptation of the virus to the drugs and even the environment temperature.
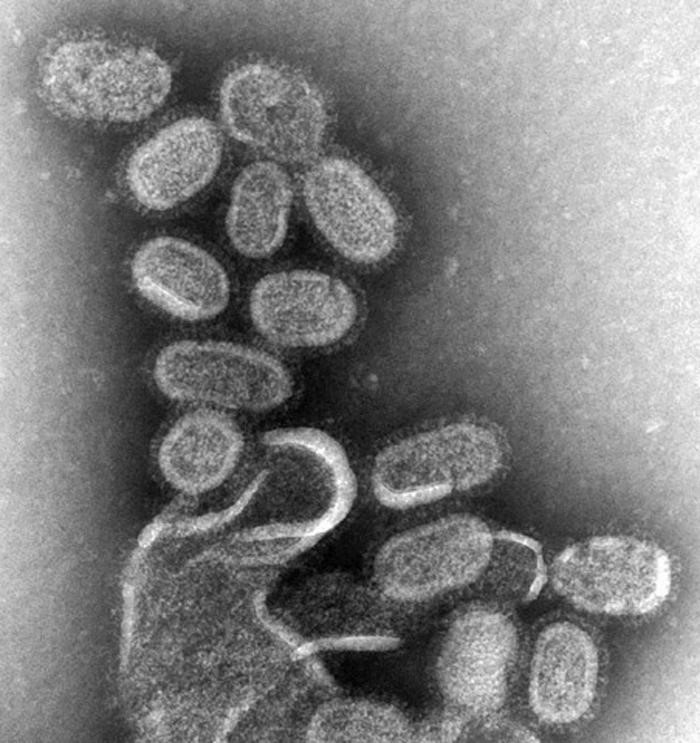
Figure 1:Influenza virus, magnified approximately 100,000 times, Specialty: Infectious disease, Symptoms: Fever, runny nose, sore throat, muscle and joint pain, headache, coughing, feeling tired, Usual onset: One to four days after exposure, Duration: 1 week, Causes: Influenza viruses.Prevention: Hand washing, influenza vaccine, surgical masks, Frequency: 3-5 million severe cases per year. Deaths: Up to 650,000 respiratory deaths per year[1-3].
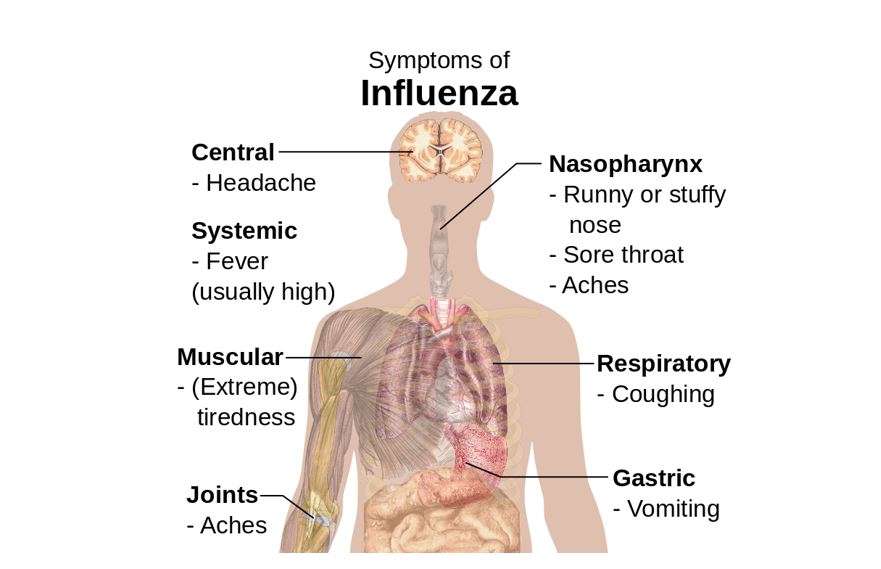
Figure 2: Symptoms of influenza, with fever and cough the most common symptoms [20-22].
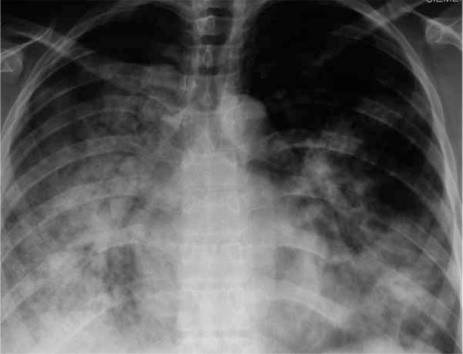
Figure 3: 29 yr. old with H1N1 with high inflammation in the lungs and respiratory systems.
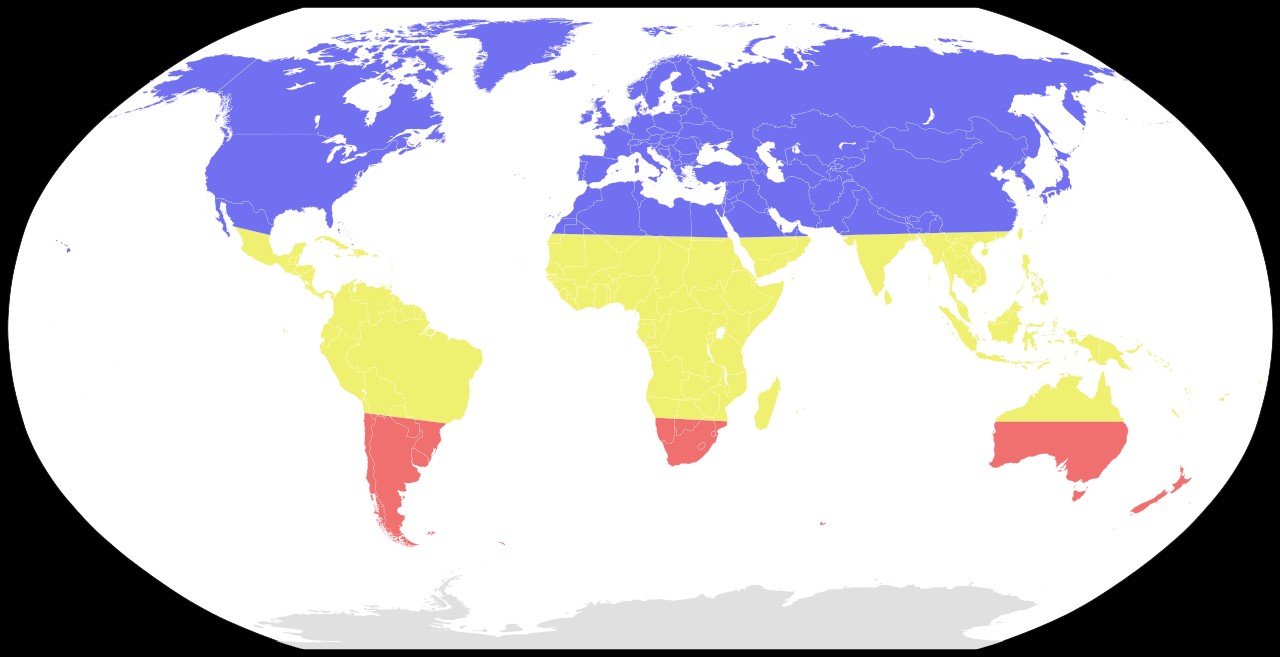
Figure 4: Seasonal risk areas for influenza: November-April (blue), April-November (red), and year-round (yellow) [21].
Figure 4:Blue-green algae, pre-date viruses and are one of the oldest forms of life on Earth.

Figure 5: The origin of the viruses was before the beginning the cellular ancestor of any species. Based on (Figure 5)and the mentioned era of the virus evolution, they have been there since 3.5 billion years ago when cyanobacteria begin to be existed. The main problem is to find what or which kinds of matter can fight this machine-like organism.
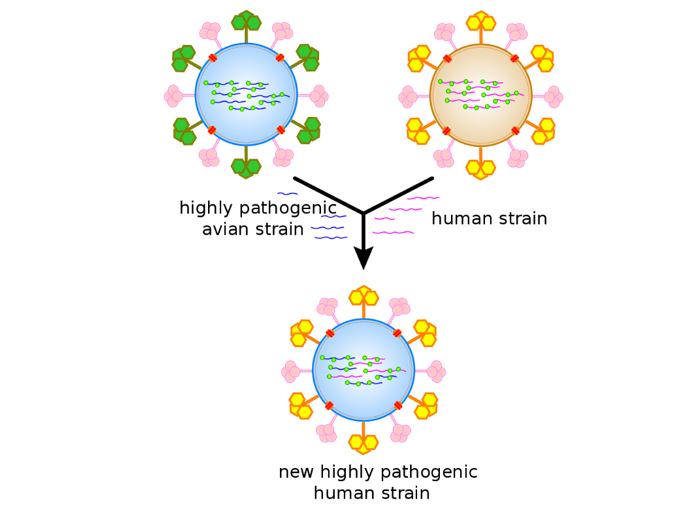
Figure 6: An antigenic shift, or Reassortment, which has the main result of novel and highly pathogenic strains of SARS-CoV-2 and other DNA and RNA viruses like All types of fatal Influenza.
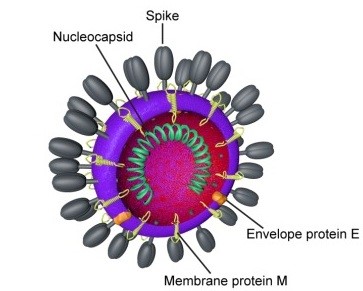
Figure 7:Diagram of coronavirus Virion structure.
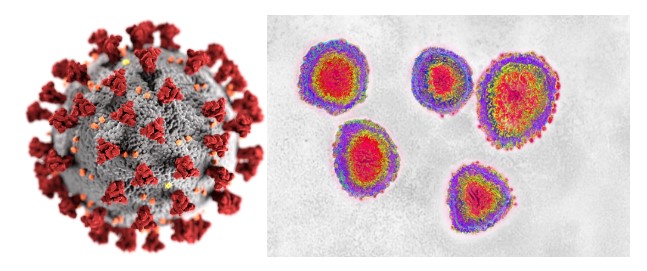
Figure 8: Illustration of a SARS-CoV-2 Virion.
Figure 9:MERS virus, Middle-East Respiratory Syndrome coronavirusVirion, (Camel Flu).
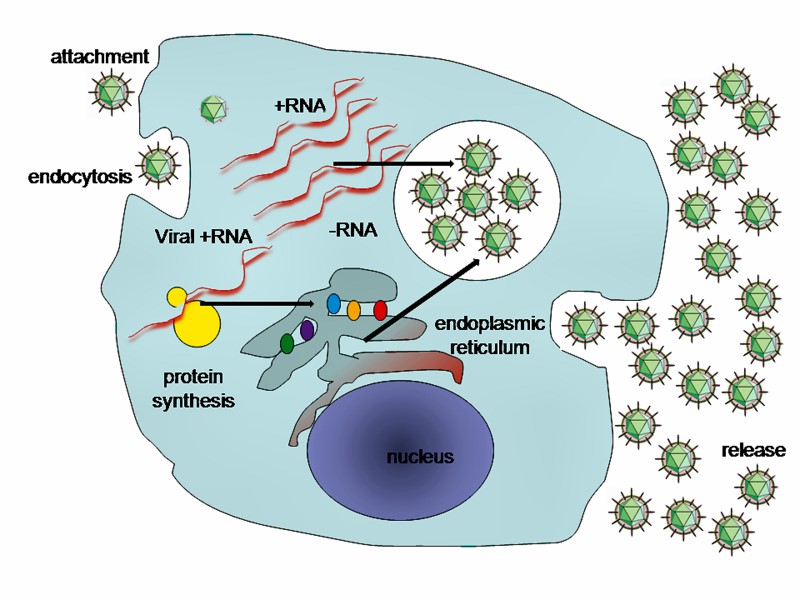
Figure 10: A typical virus replication cycleincluding Sars-Cov-2019.2019-nCoV is sufficiently divergent from SARS-CoV to be considered a new human-infecting betacoronavirus. Although our phylogenetic analysis suggests that bats might be the original host of this virus, an animal sold at the seafood market in Wuhan might represent an intermediate host facilitating the emergence of the virus in humans. Importantly, structural analysis suggests that 2019-nCoV might be able to bind to the angiotensin-converting enzyme 2 receptor in humans. The future evolution, adaptation, and spread of this virus warrant urgent investigation.

Figure 11: The bean machine, a device invented by Francis Galton, can be called the first generator of normal random variables. This machine consists of a vertical board with interleaved rows of pins. Small balls are dropped from the top and then bounce randomly left or right as they hit the pins. The balls are collected into bins at the bottom and settle down into a pattern resembling the Gaussian curve.
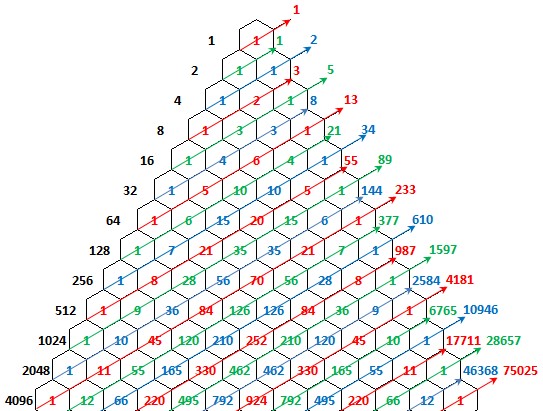
Figure 12: The right vertical numbers shows the infected people per day.
Figure 13: The number of infected people through time (day) by COVID-19.
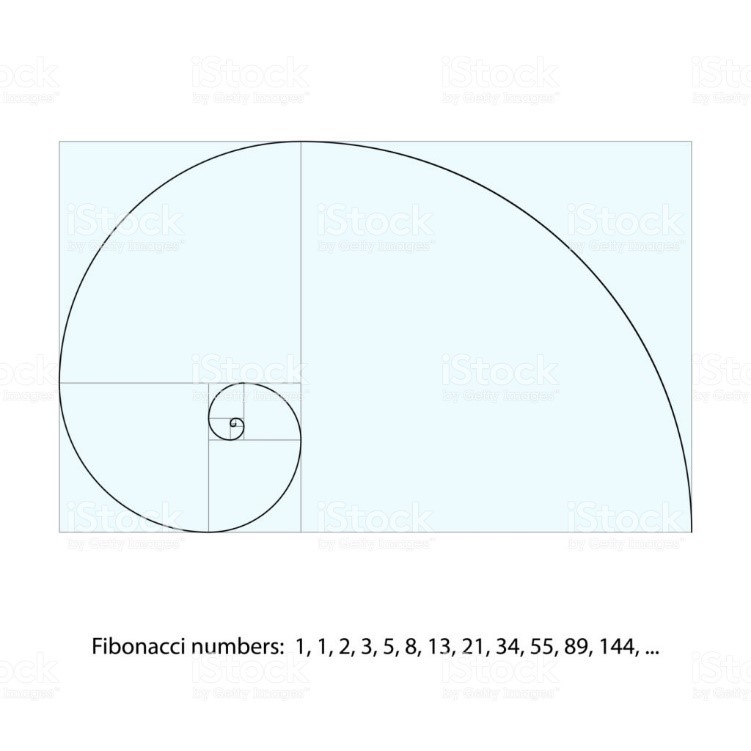
Figure 14: The Graph of Fibonacci and Pandemic of COVID-19.
|
Symptom |
Sensitivity |
Specificity |
|
Fever |
68-86% |
25-73% |
|
Cough |
84-98% |
7-29% |
|
Nasal congestion |
68-91% |
19-41% |
|
Note: All three findings, especially fever, were less sensitive in people over 60 years of age. |
||
Table 1:Most sensitive symptoms for diagnosing influenza [1-3].
|
Death toll (estimate) |
Location |
Date |
Event |
Disease |
|
75,000-100,000 |
Greece |
429-426 BC |
Plague of Athens |
Unknown, possibly typhus, typhoid fever or viral hemorrhagic fever |
|
5-10 million |
Roman Empire |
165-180 (possibly up to 190) |
Antonine Plague |
Unknown, possibly smallpox |
|
|
Europe |
250-266 |
Plague of Cyprian |
Unknown, possibly smallpox |
|
25-50 million; 40% of population |
Europe, Egypt and West Asia |
541-542 |
Plague of Justinian |
Plague |
|
|
Rome |
590 |
Roman Plague of 590 |
Plague |
|
> 100,000 |
Ctesiphon, Persia |
627-628 |
Plague of Sheroe |
Plague |
|
|
British Isles |
664-689 |
Plague of 664 |
Plague |
|
|
Japan |
735-737 |
735-737 Japanese smallpox epidemic |
Smallpox |
|
|
Byzantine Empire, West Asia, Africa |
746-747 |
Plague of 746-747 |
Plague |
|
20-200 million; 20-60% of European population |
Europe, Asia and North Africa |
1331-1353 |
Black Death |
Plague |
|
Y. pestis |
||||
|
> 10,000 |
England and later continental Europe |
1485-1551 |
Sweating sickness (multiple outbreaks) |
Unknown, possibly an unknown species of hantavirus |
Table 2: 15th century and earlier.
|
Death toll (estimate) |
Location |
Date |
Event |
Disease |
Ref. |
|
5-15 million (80% of population) |
Mexico |
1545-1548 |
Cocoliztli Epidemic of 1545-1548 |
Possibly Salmonella enterica |
[50-53] |
|
2-2.5 million (50% of population) |
Mexico |
1576-1580 |
Cocoliztli epidemic of 1576 |
Possibly Salmonella enterica |
[50-53] |
|
280,000 |
Italy |
1629-1631 |
Italian plague of 1629-1631 |
Plague |
[54-60] |
|
100,000 |
England |
1665-1666 |
Great Plague of London |
Plague |
[61-67] |
|
76,000 |
Austria |
1679 |
Great Plague of Vienna |
Plague |
- |
|
40,000 |
France |
1668 |
|
Plague |
[68] |
|
30-90% of population |
Southern New England, especially the Wampanoag people |
1616-1619 |
1616 New England epidemic |
Unknown cause. Latest research suggests epidemic(s) of leptospirosis with Weil syndrome. Classic explanations include yellow fever, bubonic plague, influenza, smallpox, chickenpox, typhus, and syndemic infection of hepatitis B and hepatitis D. |
[58-59] |
|
24,148[32] |
Netherlands |
1663-1664 |
- |
Plague |
- |
|
> 20,100 in London |
London |
1563-1564 |
1563 London plague |
Plague |
- |
|
> 19,900 in London and outer parishes |
London |
1592-1593 |
1592-93 London plague |
Plague |
- |
|
Seneca nation |
1592-1596 |
- |
Measles |
[54] |
|
|
Spain |
1596-1602 |
- |
Plague |
[55] |
|
|
South America |
1600-1650 |
- |
Malaria |
[56] |
|
|
England |
1603 |
- |
Plague |
[57] |
|
|
Egypt |
1609 |
- |
Plague |
- |
|
|
Wyandot people |
1634 |
- |
Smallpox |
[61] |
|
|
Thirteen Colonies |
1633 |
Massachusetts smallpox epidemic |
Smallpox |
- |
|
|
England |
1636 |
|
Plague |
[62] |
|
|
China |
1641-1644 |
|
Plague |
[63] |
|
|
Spain |
1647-1652 |
Great Plague of Seville |
Plague |
|
|
|
Central America |
1648 |
|
Yellow fever |
[64] |
|
|
Italy |
1656 |
Naples Plague |
Plague |
[65] |
|
|
Thirteen Colonies |
1657 |
- |
Measles |
[61] |
|
|
Spain |
1676-1685 |
- |
Plague |
[67] |
|
|
Thirteen Colonies |
1687 |
- |
Measles |
[68] |
|
|
Thirteen Colonies |
1690 |
- |
Yellow fever |
- |
Table 3: 16-17th centuries.
|
- |
Canada, New France |
1702-1703 |
- |
Smallpox |
[69] |
|
> 18,000 (36% of population) |
Iceland |
1707-1709 |
Great Smallpox Epidemic |
Smallpox |
- |
|
|
Denmark,Sweden |
1710-1712 |
Great Northern War plague outbreak |
Plague |
- |
|
|
Thirteen Colonies |
1713-1715 |
- |
Measles |
[70] |
|
|
Canada, New France |
1714-1715 |
- |
Measles |
[71] |
|
|
France |
1720-1722 |
Great Plague of Marseille |
Plague |
[72] |
|
|
Thirteen Colonies |
1721-1722 |
- |
Smallpox |
[73] |
|
|
Thirteen Colonies |
1729 |
- |
Measles |
[74] |
|
|
Spain |
1730 |
- |
Yellow fever |
|
|
|
Thirteen Colonies |
1732-1733 |
- |
Influenza |
[75] |
|
|
Canada, New France |
1733 |
- |
Smallpox |
[76] |
|
> 50,000 |
Balkans |
1738 |
Great Plague of 1738 |
Plague |
|
|
|
Thirteen Colonies |
1738 |
- |
Smallpox |
[77] |
|
|
Thirteen Colonies |
1739-1740 |
- |
Measles |
|
|
|
Italy |
1743 |
- |
Plague |
[78] |
|
|
Thirteen Colonies |
1747 |
- |
Measles |
|
|
|
North America |
1755-1756 |
- |
Smallpox |
|
|
|
North America |
1759 |
- |
Measles |
[79] |
|
|
North America, West Indies |
1761 |
- |
Influenza |
|
|
|
North America, present-day Pittsburgh area. |
1763 |
- |
Smallpox |
[80] |
|
> 50,000 |
Russia |
1770-1772 |
Russian plague of 1770-1772 |
Plague |
|
|
|
Pacific Northwest natives |
1770s |
- |
Smallpox |
[81] |
|
|
North America |
1772 |
- |
Measles |
|
|
> 2,000,000 |
Persia |
1772 |
1772 Persia plague |
Plague |
[8] |
|
|
England |
1775-1776 |
|
Influenza |
[82] |
|
|
Spain |
1778 |
- |
Dengue fever |
[83] |
|
|
Plains Indians |
1780-1782 |
North American smallpox epidemic |
Smallpox |
[84] |
|
|
Pueblo Indians |
1788 |
- |
Smallpox |
[85] |
|
|
United States |
1788 |
- |
Measles |
|
|
|
New South Wales, Australia |
1789-1790 |
- |
Smallpox |
[86] |
|
|
United States |
1793 |
- |
Influenza and Epidemic Typhus |
|
|
|
United States |
1793-1798 |
Yellow Fever Epidemic of 1793, resurgences |
Yellow fever |
[87] |
Table 4: 18thcentury.
|
Death toll (estimate) |
Location |
Date |
Event |
Disease |
Ref. |
|
1,000,000 |
Russia |
1852-1860 |
Third cholera pandemic |
Cholera |
[88] |
|
1,000,000 |
Worldwide |
1889-1890 |
1889-1890 flu pandemic |
Influenza |
[83] |
|
> 100,000 |
Asia, Europe |
1816-1826 |
First cholera pandemic |
Cholera |
[89] |
|
> 100,000 |
Asia, Europe, North America |
1829-1851 |
Second cholera pandemic |
Cholera |
[89] |
|
40,000 |
Fiji |
1875 |
1875 Fiji Measles outbreak |
Measles |
[81] |
|
> 20,000 |
Canada |
1847-1848 |
Typhus epidemic of 1847 |
Epidemic typhus |
[69] |
|
> 9,000 |
India, Germany |
1881-1896 |
Fifth cholera pandemic |
Cholera |
[89] |
|
4,737 |
Copenhagen, Denmark |
1853 |
Cholera epidemic of Copenhagen 1853 |
Cholera |
[73] |
|
3,164 |
Montreal |
1885 |
- |
Smallpox |
Timeline |
|
> 3,000 |
Central Coast, British Columbia |
1862-1863 |
- |
Smallpox |
[78] |
|
616 |
England |
1854 |
Broad Street cholera outbreak |
Cholera |
[74] |
|
Spain |
1800-1803 |
|
Yellow fever |
[86] |
|
|
Ottoman Empire, Egypt |
1801 |
- |
Bubonic plague |
[87] |
|
|
United States |
1803 |
- |
Yellow fever |
- |
|
|
Egypt |
1812 |
- |
Plague |
- |
|
|
Ottoman Empire |
1812 |
- |
Plague |
[88] |
|
|
Malta |
1813 |
- |
Plague |
- |
|
|
Romania |
1813 |
Caragea's plague |
Plague |
- |
|
|
Ireland |
1816-1819 |
- |
Typhus |
- |
|
|
United States |
1820-1823 |
- |
Yellow fever |
- |
|
|
Spain |
1821 |
|
Yellow fever |
[90] |
|
|
New South Wales, Australia |
1828 |
- |
Smallpox |
[91] |
|
|
Netherlands |
1829 |
Groningen epidemic |
Malaria |
- |
|
|
South Australia |
1829 |
- |
Smallpox |
[92] |
|
|
Iran |
1829-1835 |
- |
Bubonic plague |
[93] |
|
|
Egypt |
1831 |
- |
Cholera |
[94,95] |
|
|
Plains Indians |
1831-1834 |
- |
Smallpox |
- |
|
|
England, France |
1832 |
- |
Cholera |
- |
|
|
North America |
1832 |
- |
Cholera |
[96] |
|
|
United States |
1833 |
- |
Cholera |
- |
|
|
United States |
1834 |
- |
Cholera |
- |
|
|
Egypt |
1834-1836 |
|
Bubonic plague |
[96,97] |
|
|
United States |
1837 |
- |
Typhus |
- |
|
|
Great Plains |
1837-1838 |
1837-38 smallpox epidemic |
Smallpox |
[98] |
|
|
Dalmatia |
1840 |
- |
Plague |
- |
|
|
South Africa |
1840 |
- |
Smallpox |
- |
|
|
United States |
1841 |
- |
Yellow fever |
- |
|
|
United States |
1847 |
- |
Yellow fever |
- |
|
|
Worldwide |
1847-1848 |
- |
Influenza |
[99] |
|
|
Egypt |
1848 |
- |
Cholera |
[96,97] |
|
|
North America |
1848-1849 |
- |
Cholera |
- |
|
|
United States |
1850 |
- |
Yellow fever |
- |
|
|
North America |
1850-1851 |
- |
Influenza |
- |
|
|
United States |
1851 |
- |
Cholera |
[100] |
|
|
United States |
1852 |
- |
Yellow fever |
|
|
|
Ottoman Empire |
1853 |
- |
Plague |
[101] |
|
|
United States |
1855 |
- |
Yellow fever |
|
|
|
Worldwide |
1855-1960 |
Third plague pandemic |
Bubonic plague |
[102] |
|
|
Portugal |
1857 |
- |
Yellow fever |
|
|
|
Victoria, Australia |
1857 |
- |
Smallpox |
[103] |
|
|
Europe, North America, South America |
1857-1859 |
- |
Influenza |
[104-111] |
|
|
Middle East |
1863-1879 |
Fourth cholera pandemic |
Cholera |
[112] |
|
|
Egypt |
1865 |
- |
Cholera |
[96,97] |
|
|
Russia, Germany |
1866-1867 |
- |
Cholera |
- |
|
|
Australia |
1867 |
- |
Measles |
- |
|
|
Iraq |
1867 |
|
Plague |
[105] |
|
|
Argentina |
1852-1871 |
- |
Yellow fever |
[106] |
|
|
Germany |
1870-1871 |
- |
Smallpox |
- |
|
|
Russian Empire |
1877 |
- |
Plague |
[108] |
|
|
Egypt |
1881 |
- |
Cholera |
[96,97] |
|
|
West Africa |
1900 |
- |
Yellow fever |
|
Table 5:19thcentury.
|
Death toll (estimate) |
Location |
Date |
Event |
Disease |
Ref. |
|
|
Congo Basin |
1896-1906 |
|
Trypanosomiasis |
[109] |
|
> 800,000 |
Europe, Asia, Africa |
1899-1923 |
Sixth cholera pandemic |
Cholera |
[85] |
|
113 |
San Francisco |
1900-1904 |
- |
Bubonic plague |
[110] |
|
|
Uganda |
1900-1920 |
- |
Trypanosomiasis |
[111] |
|
|
Egypt |
1902 |
- |
Cholera |
[96,97] |
|
22 |
India |
1903 |
- |
Bubonic Plague |
[112] |
|
4 |
Fremantle |
1903 |
- |
Bubonic plague |
[113] |
|
40,000 |
China |
1910-1912 |
1910 China plague |
Bubonic plague |
[114] |
|
1.5 million |
worldwide |
1915-1926 |
1915 Encephalitis lethargica pandemic |
Encephalitis lethargica |
[115] |
|
up to 100,000,000 |
worldwide |
1918-1920 |
Spanish flu |
Influenza Spanish Flu Virus |
[116] |
|
|
Russia |
1918-1922 |
- |
Typhus |
- |
|
30 |
Los Angeles |
1924 |
1924 Los Angeles pneumonic plague outbreak |
Pneumonic plague |
- |
|
43 |
Croydon, UK |
1937 |
Croydon epidemic of typhoid fever |
Typhoid fever |
[117] |
|
- |
Egypt |
1942-1944 |
- |
Malaria |
[96,97] |
|
- |
China |
1946 |
- |
Bubonic plague |
|
|
- |
Egypt |
1946 |
- |
Relapsing fever |
[96,97] |
|
- |
Egypt |
1947 |
- |
Cholera |
[96,97] |
|
2,000,000 |
worldwide |
1957-1958 |
Asian flu |
Influenza |
[118] |
|
|
worldwide |
1961-1975 |
Seventh cholera pandemic |
Cholera |
[110] |
|
4 |
Sweden |
1963 |
|
Smallpox |
[119,120] |
|
1,000,000 |
worldwide |
1968-1969 |
Hong Kong flu |
Influenza |
[118] |
|
5 |
Netherlands |
1971 |
|
Poliomyelitis |
[121] |
|
35 |
Yugoslavia |
1972 |
1972 outbreak of smallpox in Yugoslavia |
Smallpox |
- |
|
|
United States |
1972-1973 |
London flu |
Influenza |
[122] |
|
15,000 |
India |
1974 |
1974 smallpox epidemic of India |
Smallpox |
[123] |
|
> 32,000,000 |
worldwide(commenced in Congo Basin) |
1960-present |
HIV/AIDS pandemic |
HIV/AIDS |
[124] |
|
|
South America |
1990s |
- |
Cholera |
- |
|
52 |
India |
1994 |
1994 plague epidemic in Surat |
Plague |
[125] |
|
231 |
worldwide |
1996-2001 |
- |
vCJD |
- |
|
- |
West Africa |
1996 |
- |
Meningitis |
- |
|
105 |
Malaysia |
1998-1999 |
1998-99 Malaysia Nipah virus outbreak |
Nipah virus infection |
[126] |
|
|
Central America |
2000 |
- |
Dengue fever |
[127] |
Table 6:20thcentury.
|
Death toll (estimate) |
Location |
Date |
Event |
Disease |
Ref. |
|
> 400 |
Nigeria |
2001 |
- |
Cholera |
[128] |
|
|
South Africa |
2001 |
- |
Cholera |
[129] |
|
349 |
China |
2002-2004 |
SARS outbreak |
SARS coronavirus |
[130] |
|
299 |
Hong Kong |
2002-2004 |
SARS outbreak |
SARS coronavirus |
[131] |
|
37 |
Taiwan |
2002-2004 |
SARS outbreak |
SARS coronavirus |
[132] |
|
44 |
Canada |
2002-2004 |
SARS outbreak |
SARS coronavirus |
[133] |
|
33 |
Singapore |
2002-2004 |
SARS outbreak |
SARS coronavirus |
[134] |
|
- |
Algeria |
2003 |
- |
Plague |
[135] |
|
- |
Afghanistan |
2004 |
- |
Leishmaniasis |
[136] |
|
- |
Bangladesh |
2004 |
- |
Cholera |
[137] |
|
- |
Indonesia |
2004 |
- |
Dengue fever |
[138] |
|
- |
Senegal |
2004 |
- |
Cholera |
[139] |
|
7 |
Sudan |
2004 |
- |
Ebola |
[140] |
|
|
Mali |
2005 |
|
Yellow fever |
[141] |
|
27 |
Singapore |
2005 |
2005 dengue outbreak in Singapore |
Dengue fever |
[142] |
|
|
Luanda, Angola |
2006 |
- |
Cholera |
[143-166] |
|
61 |
Ituri Province, Democratic Republic of the Congo |
2006 |
- |
Plague |
[167,168] |
|
17 |
India |
2006 |
- |
Malaria |
[169] |
|
> 50 |
India |
2006 |
2006 dengue outbreak in India |
Dengue fever |
[169] |
|
|
India |
2006 |
Chikungunya outbreaks |
Chikungunya virus |
[170] |
|
> 50 |
Pakistan |
2006 |
2006 dengue outbreak in Pakistan |
Dengue fever |
[171] |
|
|
Philippines |
2006 |
- |
Dengue fever |
[172] |
|
187 |
Democratic Republic of the Congo |
2007 |
Mwekaebola epidemic |
Ebola |
[173] |
|
|
Ethiopia |
2007 |
- |
Cholera |
[174] |
|
49 |
India |
2008 |
- |
Cholera |
[175] |
|
10 |
Iraq |
2007 |
2007 Iraq cholera outbreak |
Cholera |
[176] |
|
|
Nigeria |
2007 |
- |
Poliomyelitis |
[177] |
|
|
Puerto Rico; Dominican Republic; Mexico |
2007 |
- |
Dengue fever |
[178] |
|
|
Somalia |
2007 |
- |
Cholera |
[179] |
|
37 |
Uganda |
2007 |
- |
Ebola |
[180] |
|
- |
Vietnam |
2007 |
- |
Cholera |
[181] |
|
- |
Brazil |
2008 |
- |
Dengue fever |
[182] |
|
- |
Cambodia |
2008 |
- |
Dengue fever |
[183] |
|
- |
Chad |
2008 |
- |
Cholera |
[184] |
|
- |
China |
2008-2017 |
- |
Hand, foot and mouth disease |
[185] |
|
- |
Madagascar |
2008 |
- |
Bubonic plague |
[186] |
|
- |
Philippines |
2008 |
- |
Dengue fever |
[187] |
|
- |
Vietnam |
2008 |
- |
Cholera |
[188] |
|
4,293 |
Zimbabwe |
2008-2009 |
2008-2009 Zimbabwean cholera outbreak |
Cholera |
[189] |
|
18 |
Bolivia |
2009 |
2009 Bolivian dengue fever epidemic |
Dengue fever |
[190] |
|
49 |
India |
2009 |
2009 Gujarat hepatitis outbreak |
Hepatitis B |
[191] |
|
- |
Queensland, Australia |
2009 |
- |
Dengue fever |
[192] |
|
|
Worldwide |
2009 |
Mumps outbreaks in the 2000s |
Mumps |
|
|
931 |
West Africa |
2009-2010 |
2009-2010 West African meningitis outbreak |
Meningitis |
[193] |
|
203,000 |
Worldwide |
2009 |
2009 flu pandemic |
Influenza |
[194,195] |
|
9,985 (May 2017) |
Hispaniola |
2010-present |
Haiti cholera outbreak |
Cholera |
[196,197] |
|
> 4,500 (February 2014) |
Democratic Republic of the Congo |
2011-present |
- |
Measles |
[198][199] |
|
170 |
Vietnam |
2011-present |
- |
Hand, foot and mouth disease |
[200,201] |
|
> 350 |
Pakistan |
2011 |
2011 dengue outbreak in Pakistan |
Dengue fever |
[202] |
|
171 (as of 10 January 2013[update]) |
Darfur Sudan |
2012 |
2012 yellow fever outbreak in Darfur, Sudan |
Yellow fever |
[203] |
|
862 (as of 13 January 2020[update]) |
Worldwide |
2012-present |
2012 Middle East respiratory syndrome coronavirus outbreak |
Middle East respiratory syndrome |
[204-206] |
|
142 |
Vietnam |
2013-2014 |
- |
Measles |
[207] |
|
>> 11,300 |
Worldwide, primarily concentrated in West Africa |
2013-2016 |
Ebola virus epidemic in West Africa |
Ebola virus disease, Ebola virus virion |
[208-210] |
|
183 |
Americas |
2013-2015 |
2013-14 chikungunya outbreak |
Chikungunya |
[211] |
|
40 |
Madagascar |
2014-2017 |
2014 Madagascar plague outbreak |
Bubonic plague |
[212] |
|
36 |
India |
2014-2015 |
2014 Odisha jaundice outbreak |
Primarily Hepatitis E, but also Hepatitis A |
[213] |
|
2,035 |
India |
2015 |
2015 Indian swine flu outbreak |
Influenza A virus subtype H1N1 |
[214][215][216] |
|
~53 |
Worldwide |
2015-2016 |
2015-16 Zika virus epidemic |
Zika virus |
[217] |
|
100's (as of 1 April 2016[update]) |
Africa |
2016 |
2016 yellow fever outbreak in Angola |
Yellow fever |
[218] |
|
3,886 (as of 30 November 2019[update]) |
Yemen |
2016-present |
2016-17 Yemen cholera outbreak |
Cholera |
[219] |
|
64 (as of 16 August 2017[update]) |
India |
2017 |
2017 Gorakhpur Japanese encephalitis outbreak |
Japanese encephalitis |
[220] |
|
18 (as of February 2020[update]) |
India |
2018 |
2018 Nipah virus outbreak in Kerala |
Nipah virus infection |
[221] |
|
2,253 (as of 20 February 2020[update]) |
Democratic Republic of the Congo & Uganda |
August 2018-present |
2018-19 Kivu Ebola epidemic |
Ebola virus disease |
- |
|
>6,000 (by January 2019) |
Democratic Republic of the Congo |
2019-present |
2019 measles outbreak in the Democratic Republic of the Congo |
Measles |
- |
|
83 |
Samoa |
2019-present |
2019 Samoa measles outbreak |
Measles |
- |
|
3,360 (as of 6 March 2020[update]) |
Worldwide |
2019-present |
2019-20 coronavirus outbreak |
COVID-19 |
- |
Table 7: 21st century.
Citation: Niknamian S and Zaminpira S (2020) The Historical/Evolutionary Cause and Possible Treatment of Pandemic Covid-19 (Sars-Cov-2, 2019-Coronavirus). Ann Med &Surg Case Rep: AMSCR-100050