Emerging Infectious Diseases and Diagnosis Journal
ISSN 2652-4449
Volume 02; Issue 03
Review Article
Lessons Learned from Health Disparities Among African Americans in The HIV Epidemic: What to Expect for COVID-19 and Potential Approaches to Mitigate Health Disparity
Kodidela S1*, Kumar A1, Gerth K1, Walker C2,and Kumar S1*
1Department of Pharmaceutical sciences, University of Tennessee Health Science center, Memphis, USA
2Department of Health Promotion and Disease Prevention, College of Nursing, University of Tennessee Health Science center, Memphis, USA
*Corresponding authors: Santosh Kumar, Department of Pharmaceutical sciences, University of Tennessee Health Science center, Memphis, USA.
Sunitha Kodidela, Department of Pharmaceutical sciences, University of Tennessee Health Science center, Memphis, USA.
Citation: Kodidela S, Kumar A, Gerth K, Kumar S, Walker C (2020) Lessons Learned from Health Disparities Among African Americans in The HIV Epidemic: What to Expect for COVID-19 and Potential Approaches to Mitigate Health Disparity. Emerg Infect Dis Diag J: EIDDJ-100021
Received date:11 May, 2020; Accepted date: 15 May, 2020; Published date: 22 May, 2020
Abstract
In the ongoing HIV pandemic, disaggregated data from African American (AA) communities in the United States reveal differences in the prevalence and incidence of HIV infection and AIDS diagnoses across racial groups. These differences are huge in terms of disparities in infection rates among AA compared with Caucasian Americans (CA). Several studies that have indicated differences in prevalence of infection among blacks have focused on social and/or structural factors, including socioeconomic issues associated with income or education level, housing and awareness about HIV and other sexually transmitted diseases. Also, at the individual level, personal and behavioral characteristics contribute to the influence of psychological factors, namely and racial discrimination, that may prevent AA from accessing HIV care services. All these factors likely fuel health disparities and contribute to limited prevention and reductions in HIV prevalence. Apparently, the current COVID-19 pandemic is following similar trends as HIV prevalence in AA in the United States. The evidence that AA are predisposed to higher risk and severity of COVID-19 infection is not previously documented. This is the first attempt to present the data in peer-reviewed literature on the racial distribution of COVID-19-confirmed cases in different U.S. states. The underlying mechanisms to support the epidemiological connections in AA will require further investigations. This review explores the risk factors for COVID-19 in the context of race and health disparities, as a higher prevalence exists in AA than in CA. This review also explores potential ways to mitigate COVID-19 racial disparity including potential treatment options.
Keywords:African American; Caucasian American; COVID-19; Health disparity; HIV
Historical perspective of health disparities (HD) among African American in HIV infection in USA
International travel facilitated HIV dissemination from Africa to rest of the globe in the early 1970s[1,2]. However, its impact in the United States was not realized until the early 1980s[2]. In 1980, HIV was identified among the American homosexual male population, and the general public considered it a “gay disease” and even referred to it as the "Gay Plague” for many years [3-5]. Initially, HIV infection rates were minimal in the African American (AA) community [6]. The infection was thought to spread among Caucasian Americans (CA), and it was considered a problem of white, gay men[7]. Though precautionary measures such as protective sex and avoiding direct contact with infected body fluids were put into place, increased prevalence of the injection drug use among AA and socioeconomic conditions eventually led to increased cases of HIV infection among the AA population [7-11]. Healthy People 2020 defines a health disparity (HD) as “a particular type of health difference that is closely linked with social, economic, and/or environmental disadvantages. Health disparities adversely affect groups of people who have systematically experienced greater obstacles to health, based on their racial or ethnic group; religion; socioeconomic status; gender; age; mental health; cognitive, sensory, or physical disability; sexual orientation or gender identity; geographic location; or other characteristics historically linked to discrimination or exclusion”[12]. In 2007, the US Centers for Disease Control and Prevention (CDC) published a report on health disparities in HIV/AIDS and other infections[13] based on 2000 to 2004 surveillance data[14]. This data reported that AA comprised only 13% of the US population, but accounted for half of all new HIV/AIDS diagnoses. Soon after the 2007 report from the CDC on HD, the number of studies looking at HD among AA have drastically increased (Figure 1). Though cases of new infections have decreased over time, the AA population is still disproportionately affected by HIV [15]. Further, AA are even more severely affected by HIV in the southern U.S., especially in the Delta region, accounting for 52% of new HIV diagnoses in that area[16]. The HD in HIV infection rates among AA are due to socioeconomic conditions, namely poverty, lower educational attainment, family residential instability, drug abuse, unequal access to healthcare, and lower quality of HIV care [13]. We shall describe a few of these in the context of COVID-19 in the next section.
Lessons learned from health disparities in HIV: what to expect in COVID-19
The HIV epidemic in the USA started in the 1980s, but due to insufficient knowledge about the infection rate, disease pathology, and its severity health officials didn’t recognize the potential impact of it on public health. Therefore, not much attention was paid in terms of implementing preventive measures until the late 1980s. This eventually allowed HIV to spread all over the country, predominantly affecting the AA community because of socioeconomic factors[9,17]. Apparently, a similar scenario is being observed in the COVID-19 crisis. The first case of COVID-19, which occurs as a result of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, was reported in Dec 2019 in Wuhan, China [18]. Since then, it has rapidly spread across the globe and the World Health Organization (WHO) declared COVID-19 as pandemic. The first case of COVID-19 in the USA was reported much later than in China, in February 2020[19], perhaps due to an initial lack of knowledge pertaining to the virus’s incubation time and disease progression. Therefore, no swift actions were taken until February/March 2020 to enact precautionary measures, such as social distancing, an international travel ban, etc., to curb the spread of infection. Currently, there are over 1,152,372 confirmed cases of COVID-19 and over 67,456 deaths all over the country, as of May 4th, 2020[20]. The CDC published an online report on April 8th, which considered the 1,482 coronavirus-infected patients who were hospitalized across 14 states, from March 1st to March 30th. Of these 1,482 cases, the racial data was available for 580, and revealed that AA accounted for 33% of COVID-19 hospitalizations[21]. While this manuscript is under preparation, a report on COVID 19 deaths data by race has been published. This report suggested that AAs are 2.6 times more likely to die than CAs [22]. To understand the current scenario of HD in COVID-19 cases in the AA population, we have collected COVID-19 case data from the departments of health of different US states, as of April 24th, 2020 [23]. There is a total of 895,534 infection cases, and of these, race data is being reported for 474,314 cases. Among infected cases with racial data available, CA comprise 35% and AA 23%, while the remainder is a combination of other races and unspecified categories (Figures 2). Considering that AA are a minority population in the USA(12%), 23% representation among all l COVID-19 cases is relatively high (Figure 2).
The race data of COVID-19 cases were reported by 32 states in the USA (Figure 3).
As observed in HIV infection rates, the AA population is disproportionately affected by COVID-19. While Figure 3Apresents the actual number of COVID-19 cases in AA, CA, and other populations, Figure 3Bpresents the relative COVID-19 cases per 100,000 populations in AA compared to CA populations in the US states, where the infection is relatively high. As shown in Figure 3B, the relative rate of COVID-19 cases per 100,000 populations is much higher in the AA than CA populations in all the states shown here. Although this trend is only based on approximately 40 days’ data, health disparities pertaining to COVID-19 infection will likely perpetuate in the AA population, unless appropriate measures are taken to understand underlying causes and potential remedies. This trend of HD in the AA population is even more critical in the Delta region, where the AA population is higher than the national average. The Delta region consists of eight states: Alabama, Arkansas, Illinois, Kentucky, Louisiana, Mississippi, Missouri, and Tennessee. Reported positive cases among AA in Louisiana and Mississippi are already higher than for the CA population (Figure 4, respective right bar for each state), and the number is relatively high when compared to the total AA population in the USA (Figure 4, respective left bar for each state). In general, the relative number of COVID-19 cases in the AA population in all Delta states is higher than in the CA population of those states. In Tennessee, Shelby County is the largest county by population, and as of April 26th, 2020, 2,133 confirmed cases of COVID-19 and 45 fatalities have been reported. Out of 2,133 cases, 68.1% are AA, 25.8% are CA, and 6.1% are other race[24]. These preliminary statistics suggest that HD relating to COVID-19 are much greater among the AA population than the CA population. Therefore, based on existing knowledge of HD in HIV in the last two decades, it is critically important to understand the basis for this disparity in COVID-19 infection rates in the AA population. Here, we describe the risk factors associated with COVID-19 disparities in terms of viral infection as well as disease severity in the AA population, followed by necessary steps which can be taken to reduce this, and potential therapeutic options.
Risk factors for exposure to infection among AAs:Although precautionary measures were suggested to combat the HIV epidemic, transmission rates increased in the AA population because of increased prevalence of injection drug use, and socioeconomic conditions [7-10,25]. Similarly, despite suggested preventive measures such as social distancing and hand sanitation, COVID-19 cases are on rise among AA population due to their essential worker occupation and socioeconomic conditions. These trends will continue unless we study the underlying reasons for health disparities in AA and take steps to reduce these inequities. Although many states have declared stay-at- home orders, this is not possible for people with “essential worker” occupations who cannot telecommute, such as caregivers, grocery store clerks, emergency dispatchers, and public transportation employees. AA are highly employed in these services[26,27], which makes it difficult for them to maintain effective social distancing and consequently increases the risk of COVID-19 exposure. Further, stay-at-home orders have already resulted in unemployment in certain career sectors where many AA are employed[28]. Unemployment among the AA population increases their risk of exposure due to economic consequences, such as eviction and the need to search for new jobs, often without proper protection[28].
Risk factors exacerbating disease complications among AAs.
Poverty is a significant reason for health disparities:Low average economic status among AA reduces their access to high quality food, healthcare, and safe housing[29-32]. Inadequate health insurance coverage reduces access to reduces access to HIV-related care and treatment for HIV-associated complications, such as cardiovascular disease, cancer, and other infections [32,33]. As observed in HIV HD, insufficient access to healthcare make it difficult for AA to get tested for COVID-19 and treated for COVID-19 associated complications.
Housing disparities are also a risk factor for severe complications of HIV among the AA population [32,34]: AA are more likely than CA to live in older communities and in neighborhoods with more exposure to sources of air pollution, such as highways, factories, and refineries [35,36]. These conditions make HIV infected population susceptible to various infections due to their immunocompromised status. Similarly, these conditions exacerbate asthma and other respiratory conditions, and are a major underlying risk factor for severe and potentially fatal cases of COVID-19 [37,38].
Tobacco smoking, which is known to exacerbate HIV-associated complications [39], is also a risk factor for susceptibility to developing severe complications of COVID-19[40]: Although the prevalence of cigarette smoking among AA and CA is the same, AA are more likely to die from smoking-related diseases than CA[41]. Further, smoking is also a major risk factor for developing cardiovascular disease and asthma [42,43], underlying diseases which worsen COVID-19 severity and increase a patient’s likelihood of admission to an ICU. It has been found that AA are infected with SARS-CoV-2 at higher rates and are more likely to develop severe symptoms that result in death[44].
Even after several decades of the HIV pandemic, HD persist in the present day among AA in various diseases, including HIV [45,46]. Based on the trend from HD in HIV and underlying causes, we expect a higher number of COVID-19 infections in AA than in CA. Therefore, there is a need to improve factors contributing to HD among AA by the following approaches.
Future actions to address healthcare disparities
Collaborative and integrative programs:Integrative medicine reaffirms the patient-practitioner relationship, while combining traditional healthcare with complementary and alternative medicine (CAM) approaches, e.g. including nutrition and exercise interventions for the treatment of cardiovascular disease or using mindfulness techniques for mental health conditions[47]. Use of CAM is common amongst people living with HIV/AIDS (PLWHA) and includes the use of vitamins and herbal supplements, as well as spiritual techniques such as prayer and meditation[48]. Self-management provides patients a sense of control over their health status, but clinician oversight is warranted to ensure that alternative therapies do not interfere with the efficacy of traditional treatments, e.g. drug-drug interactions (DDI) with ART[48]. However, patient use of integrative approaches is also correlated with socioeconomic factors, which influence patient awareness, availability, accessibility, and affordability of complementary and alternative regimens[47]. If integrative approaches are proposed to combat comorbidities that predispose individuals for higher morbidity and mortality from COVID-19 infection, then the availability, accessibility, and affordability of such tools to implement appropriate lifestyle modifications must also be considered. Collaborative programs may include community-level initiatives to help address healthcare disparities. For example, community health worker programs use lay people as a bridge between patients and providers. These lay workers collaborate with health departments, homeless programs, community health centers, and academic medical centers[49]. Such liaisons help increase patient access to care, aid in the delivery of culturally appropriate patient education, promote patient advocacy, improve patient attendance to medical appointments and adherence to medication regimens, and enhance the use of primary and preventative care, among other benefits[49]. Additionally, patient-centered, multidisciplinary approaches are secondary prevention strategies that use teams of physicians, nurses, dieticians, and other healthcare providers to enhance risk reduction in target populations[49]. Multidisciplinary care allows practitioners to employ cost-effective and comprehensive treatment strategies[50]. These approaches have shown promising results for the treatment of many chronic health conditions, including cardiovascular disease[51], obesity[52]and diabetes [53], among others.
Improved Testing and Screening
HIV:The CDC recommends that all adults aged 13-64 engage in routine HIV screening, with annual repeat screenings for higher risk individuals, e.g. men who have sex with men, IV drug users, those with an HIV-positive sexual partner, etc.[54]. Although an early HIV diagnosis is crucial for achieving viral suppression and reducing disease transmission, the CDC estimates that only ~40% of U.S. adults have ever been tested, based on data from 2006-2016. However, numbers for AA were higher, at 57.4%[55]. Programs like The Expanded Testing Initiative have diverted resources to screening disproportionately affected populations[56] in order to further address healthcare disparities related to HIV.
COVID-19:Although development of a vaccine for COVID-19 may take over a year[57], many U.S. states are already taking steps to re-open the economy by May 1st, 2020, despite concerns from the CDC for a second wave of infections. In an April 20threport from The Safra Center for Ethics at Harvard University, experts called for the adoption of rapid, widespread testing, along with a ‘phased mobilization’ of the economy[58]. However, testing shortages in the U.S. have greatly hindered the implementation of widespread testing. As of April 24th, the CDC reports that only 416,035 specimens have been tested for SARS-COV-2[38]. The FDA has already taken steps to accelerate the development and validation of tests[59]. Rapid, accurate testing is of the utmost importance for detecting COVID-19 cases early, before the virus is spread to others[60]. ‘Track and trace’ programs have also been proposed, by which trackers can identify where infected persons have been and with whom they have had contact[58]. Further, ‘drive thru’ testing has emerged as an effective way of minimizing contact between patients and healthcare workers, which has the additional benefit of conserving personal protective equipment (PPE) [61]. New rapid testing kits, including self-swab from home testing kits, are expected to expedite the rate of testing in the US in the next few weeks. However, it is important to note that testing centers and kits must also be easily accessible and affordable in order to address healthcare disparities and make widespread testing an attainable goal.
Effectiveness of preventative programs:Sexual health interventions, as well as other HIV prevention programs and pharmacological treatments, have shown promising results [62].While new HIV infections have declined since the 1980’s, data from 2010-2016 revealed that infection rates have since stabilized[63]. This stalled progress prompted the current administration to announce the ‘Ending the HIV Epidemic’ plan in 2019[64]. Although effective interventions have been designed and tested, their implementation has, in many cases, been offset by a lack of community resources [65]. Government action is therefore critical for wide scale implementation of evidence-based, cost-effective approaches, and the targeting of resources to high-risk populations[66]. Likewise, actions by policymakers and government bodies will determine the future success of prevention strategies for pandemics like COVID-19.
Further, in order to implement effective COVID-19 preventative strategies amongst AA, it will be crucial to increase their involvement in research and development studies, strengthen patient-provider relationships, and build trust in medical institutions. The AA community has had a complicated history with medical research, and the often-cited Tuskegee Syphilis Study is just one example of the historical basis for AA mistrust of academic and research institutions[67]. Decades later, AA remain underrepresented in clinical research[68]. In order to develop relevant therapeutics and vaccinations to combat the COVID-19 pandemic, it will be crucial to include AA in research studies so that future treatment strategies may function to attenuate health disparities rather than contributing to them. For example, the relative efficacies and adverse effects of known therapeutics can vary by ethnic group[69]. Fair representation in research studies will therefore facilitate the development of treatment strategies that do not worsen outcomes for vulnerable populations. In fact, there is a guideline from the NIH that all research activities with human subjects include an equal representation of AA and CA, especially in the case of diseases that affect AA population significantly. Moreover, once a vaccine for SARS-COV-2 is developed, there will need to be concentrated efforts to deploy the vaccine to high-risk populations. Importantly, AA are 43% less likely to get seasonal vaccinations for influenza than CA[70]. Reasons for lower vaccination rates amongst the elderly AA population are thought to extend well beyond access to care. Reports suggest that vaccination disparities are likely to involve resistant attitudes and beliefs regarding vaccinations, as well as discrimination by providers[71].
Economic considerations: The economic impact of the COVID-19 shutdown, for many minority communities, cannot be ignored. While quarantine and social distancing measures are necessary to reduce viral spreading[72], workplace closures resulted in record-high unemployment claims in March 2020[73]. According to the U.S. Department of Labor, unemployment rates rose 6.7% for AA versus 4% for CA [74]. Economic inequalities are well-documented barriers to health literacy, access to adequate healthcare and insurance coverage, access to healthy food, and chronic disease prevention [44,49,75,76]. These barriers are associated with higher rates of comorbidities that are correlated with COVID-19 morbidity and mortality[77]. Low socioeconomic status is also linked to high-risk behaviors that are associated with higher rates of HIV infection [78]. The fallout from the COVID-19 pandemic has only exacerbated such inequities. Additionally, those who live in areas of high density housing and essential workers who are unable to work from home are at a higher risk of infection even during the shutdown, due to an inability to achieve true social distancing [44].
Thus, while health officials and researchers can pave the way for reducing healthcare disparities and containing the spread of infectious diseases, ultimately, the relative effectiveness of these actions is in the hands of policymakers. Improved testing strategies, as well as collaborative programs and adequate preventative care are only parts of the equation for mitigating viral spread. The development, validation, and appropriate utilization of pharmacological therapies and vaccines should also consider underlying conditions or health disparities amongst racial groups, if these measures are to be effective and lasting. Therefore, we describe the currently available therapeutic options for severe and hospitalized COVID-19 patients with underlying conditions, which are relatively high in AA population.
Potential therapeutic options for COVID-19
The impact of COVID-19 is more extreme in people with suppressed immune systems and in patients with preexisting diseases such as diabetes, hypertension, and cardiovascular disease[79], which are more prevalent in AA than in CA populations[45]. Although further studies are required to establish the severity and mortality factors associated with COVID-19, new concerns have arisen based on early evidence of potentially egregious health care disparities among the AA population. The CDC released updated preliminary nationwide data on April 18th, 2020, which suggested that COVID-19 is having a disproportionate impact on AA as compared to the CA population [38]. This disproportionality in COVID-19 morbidity and mortality could be correlated with higher rates of pre-exiting conditions such as obesity, high blood pressure, and diabetes among AA [45]. At present, there are no drugs or other therapeutics approved by the USFDA to alleviate the severity and improve the prognosis of patients with COVID?19[80]. With the rapid and widespread transmission of SARS-Cov-2 in the United States, researchers had started testing a following range of therapies.
Chloroquine and Hydroxychloroquine:Chloroquine and its derivative hydroxychloroquine initially drew much attention from researchers due to their proven anti-viral and anti-inflammatory effects [81]. Studies, mostly in vitro, have shown the potential of Chloroquine and hydroxychloroquine against viruses, including coronaviruses[82-84], HIV[85,86]and influenza[87]. In the early research findings, chloroquine had been reported as a potential anti-viral drug that could block SARS-Cov-2 infection at low-micromolar concentrations in human cells in vitro[88,89]. Chloroquine and hydroxychloroquine are known to increase endosomal pH, resulting in inhibition of autophagosome-lysosome fusion and inactivating enzymes necessary for viral replication[90,91], as well as interfere with terminal glycosylation of the angiotensin-converting enzyme 2 (ACE2), a cellular receptor of SARS-Cov[92,93]. Studies from China showed benefits of chloroquine in terms of reducing the severity of pneumonia in patients with COVID-19, duration of symptoms and delay of viral clearance in clinical trials [94]. However, due to methodological limitations and small sample sizes of these studies, it is too early to conclude that there is a significant benefit.
Despite limited information known about the safety and effectiveness of these drugs, the USFDA issued an Emergency Use Authorization (EUA) to allow the emergency use of chloroquine phosphate and hydroxychloroquine sulfate for the treatment of COVID-19 in hospitalized adults and adolescents over 50 kg[95,96]. However, the FDA reviewed case reports concerning serious heart-related adverse events and mortality in patients with COVID-19 treated with chloroquine and hydroxychloroquine, either alone or in combination with the antibiotic azithromycin and other QT prolonging medicines[97]. Patients who also have other health issues such as heart and kidney disease are likely to be at increased risk of these heart problems when receiving these medicines.
Hence, the FDA issued a warning on April 24th, 2020 against taking these drugs outside a hospital setting and recommended initial evaluations and monitoring, including baseline ECG, electrolytes, renal function and hepatic tests, when using these drugs under the EUA[98]. Since AAs are reported to have higher rates of pre-exiting conditions such as obesity, high blood pressure, and diabetes[45], these drugs should be prescribed cautiously in this population. Further, hydroxychloroquine caused a severe hemolytic crisis in a COVID-19 patient with glucose-6-phosphate dehydrogenase (G6PD) deficiency[99]. Therefore, special care must be taken before considering chloroquine and hydroxychloroquine for patients with COVID-19, especially in the African American population, where the prevalence of G6PD deficiency is estimated to be around 11-13%[100]. The possible reason for unfavorable/worse outcomes with these drugs is owing to drug-mediated toxicity[101]. Therefore, the importance of drug-drug interaction studies should be highlighted, and clinicians should await the results of ongoing prospective, randomized, controlled clinical trials, before widespread adoption of these drugs for treating COVID-19.
Remdesivir: Remdesivir is an antiviral drug currently under investigation for its potential use in the treatment of COVID-19. Remdesivir is an adenosine analogue that works by inhibiting RNA-dependent RNA polymerase that RNA viruses, including SARS-CoV-2, use to replicate in the host cells [102-104]. The drug showed broad-spectrum antiviral activity both in vitro and in vivo in pathogenic animals, against multiple emerging viral pathogens, including Ebola, Marburg, MERS-Cov and SARS-Cov [105-107].According to the study published on Feb 4th, 2020, remdesivir showed its potency to block SARS-Cov-2 viral infection in human cells in vitro [88]. Beginning on February 21st, a randomized, placebo-controlled clinical trial, formally called the Adaptive COVID-19 Treatment Trial, evaluated the safety and efficacy of remdesivir in 1,063 hospitalized U.S. patients [108]. Hopes rose for remdesivir after Anthony Fauci, director of the NIAID, announced preliminary results from the clinical trial on April 29, indicating that recovery time for patients who received remdesivir was significantly improved compared with those who received placebo (p<0.001). Specifically, time to recovery was reduced to 11 days for patients who received remdesivir, compared with 15 days for those who received placebo. The mortality rate was also investigated and was found to be 8% for the remdesivir group, compared to 11.6% for the placebo group (p=0.059)[108]. However, the difference in mortality rate was not found to be statistically significant in the preliminary analysis. Nevertheless, remdesivir seems to be well-tolerated in hospitalized patients with severe manifestations of COVID-19 disease, during the open-label phase 3 trial study[108].
Outside the U.S. trial, China found no statistically significant difference in clinical recovery rate between remdesivir when compared with a placebo group in a small multicenter, double-blind, placebo-controlled, trial involving 237 patients (158 for remdesivir and 79 for placebo group)[109. Nevertheless, researchers are looking positively at the NIAID clinical trial, having a large sample size and tightly regulated data that was overseen by an independent data and safety monitoring board (DSMB). On May 1st, 2020, based on the totality of scientific evidence available, USFDA granted Gilead Sciences the authorization of emergency use of remdesivir for the treatment of COVID-19[95]. Additional investigation is warranted to evaluate the further safety and efficacy of the drug, as remdesivir is not yet licensed or approved and has not yet been demonstrated to be safe for the treatment of COVID-19.
Compared to chloroquine and hydroxychloroquine, one advantage of remdesivir is that it is given as intravenous injection in controlled hospital environment, thus reducing the probability of severe adverse events and death caused by remdesivir in the clinical setting. In contrast, since remdesivir acts at the early stage of viral replication (RNA polymerization), it may make remdesivir relatively more effective at early than late stage of infection. However, since remdesivir is not given orally as tablet, it is hard to test this hypothesis with patients at early stage of the infection. It may be worth formulating remdesivir as tablet followed by its clinical trial with patients during early stage of infection.
Other options:An early randomized, controlled, open-label trial involving hospitalized adult patients with COVID-19 in a small sample size, showed no benefit when patients received a combination of two antiretroviral drugs, lopinavir–ritonavir [1101]. However, a study reported in vitro antiviral activity of lopinavir and ribavirin against SARS-Co-2, and reduced viral load and disease severity with combination of lopinavir–ritonavir in patients with SARS[111]. Further, comprehensive molecular dynamics studies have shown strong interaction between these as well as other antiviral protease inhibitors and SARS-Cov-2 protease[112]. Therefore, antiviral protease inhibitors have been proposed to encapsulate in biological nanoparticles called extracellular vesicles and target the drugs in lung cells to enhance their efficacy and reduce off-target effects[104]. COVID-19 severity is known to be associated with cytokine storms[113], though further research is warranted to know the spectrum and pathophysiology of COVID-19. Interleukin-6 (IL-6) plays an important role in virally driven inflammation[114,115]. Preliminary studies from China have shown that neutralizing cytokine storms helps to reduce deaths in severe cases of Covid-19 [116]. A multicenter, randomized controlled trial of tocilizumab (IL-6 receptor inhibitor), to evaluate the safety and efficacy of tocilizumab in the treatment of COVID-19, has been initialized [117]. Moreover, a preliminary report on 10 Chinese patients with COVID-19 showed clinical benefit after receiving convalescent plasma therapy with no serious adverse effects [118]. However, as of May 1, 2020, the FDA has not approved convalescent plasma for use in patients with COVID-19, and it is regulated as an investigational therapy [119]. The FDA issued guidance to provide recommendations to clinicians and researchers before considering and studying investigational convalescent plasma obtained from individuals who have recovered from COVID-19 during the pandemic.
Conclusion
Lessons learned from the HD among AA in the HIV epidemic suggest that the racial disparity is likely to emerge in the COVID-19 pandemic. Our review of the preliminary data demonstrates that the AA population has a higher rate of COVID-19 in comparison to their respective population percentage in the USA. Since healthcare disparities between African Americans and the general population are multifactorial in origin, it is essential that any future actions to address the prevalence of infectious diseases, including HIV and COVID-19, must be holistic in nature. Improved testing strategies, the development, validation, and appropriate utilization of pharmacological therapies and vaccines, as well as collaborative programs and adequate preventative care, are only parts of the equation for mitigating viral spread. Each must be viewed in the context of underlying socioeconomic inequalities amongst racial groups, if these measures are to be effective and lasting.
Funding:This study is supported by the funding opportunity from the National Institute of Health (DA047178).
Conflict of interest: Authors declare no conflict of interest.
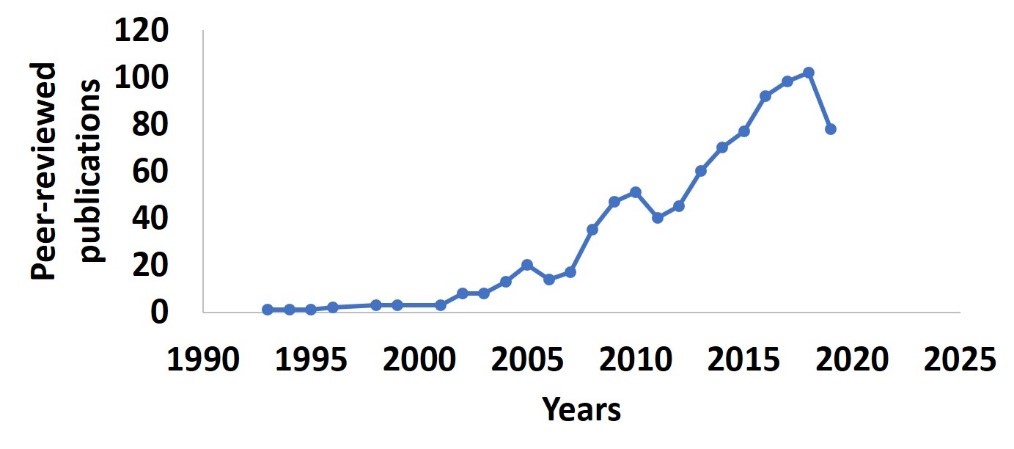
Figure 1:Peer-reviewed articles studying health disparities in African American population infected with HIV published in PubMed until Dec 31st2019. The search terms used are “Health disparity AND African American AND HIV”.
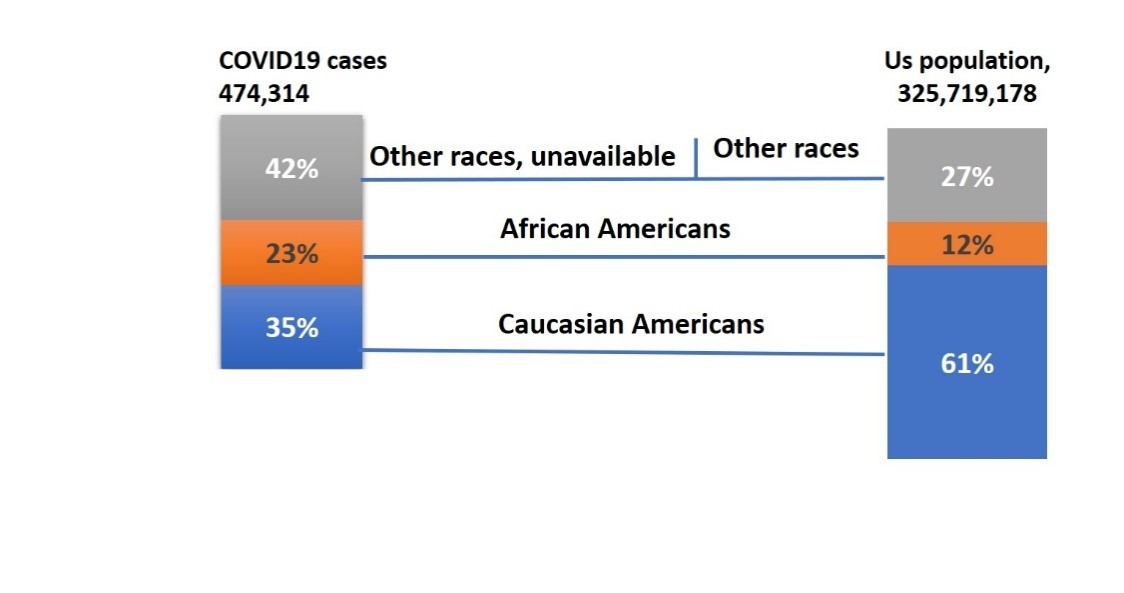
Figure 2: Estimated COVID19 Diagnoses by race as of April 24th 2020 & The US Population by Race/Ethnicity, 2017.Race data of COVID19 was reported by 32 states mentioned below in the USA. Thus, the respective population of those 32 states has been considered. Alabama,Arizona,Arkansas,California,Connecticut, District of Columbia, Georgia, Illinosis, Indiana, Iowa,Louisiana,Maryland,Massachusetts,Michigan,Minnesota,Mississippi,Missouri,NewJersey,NewMexico,North Carolina,Oklahoma,Oregon,Rhode Island,South Carolina,Tennessee,Texas,Utah,Virginia,Washington,West Virginia,Wisconsin,Wyoming.
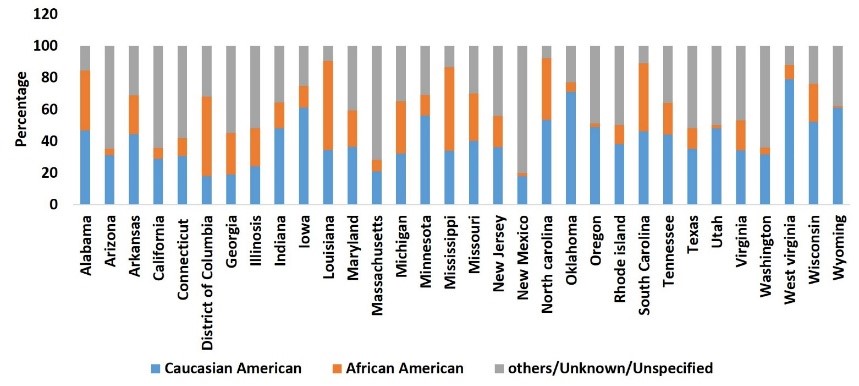
Figure 3A:Percentage distribution of COVID-19 cases by race(n=474,314) in the USA as of 24thApril 2020.
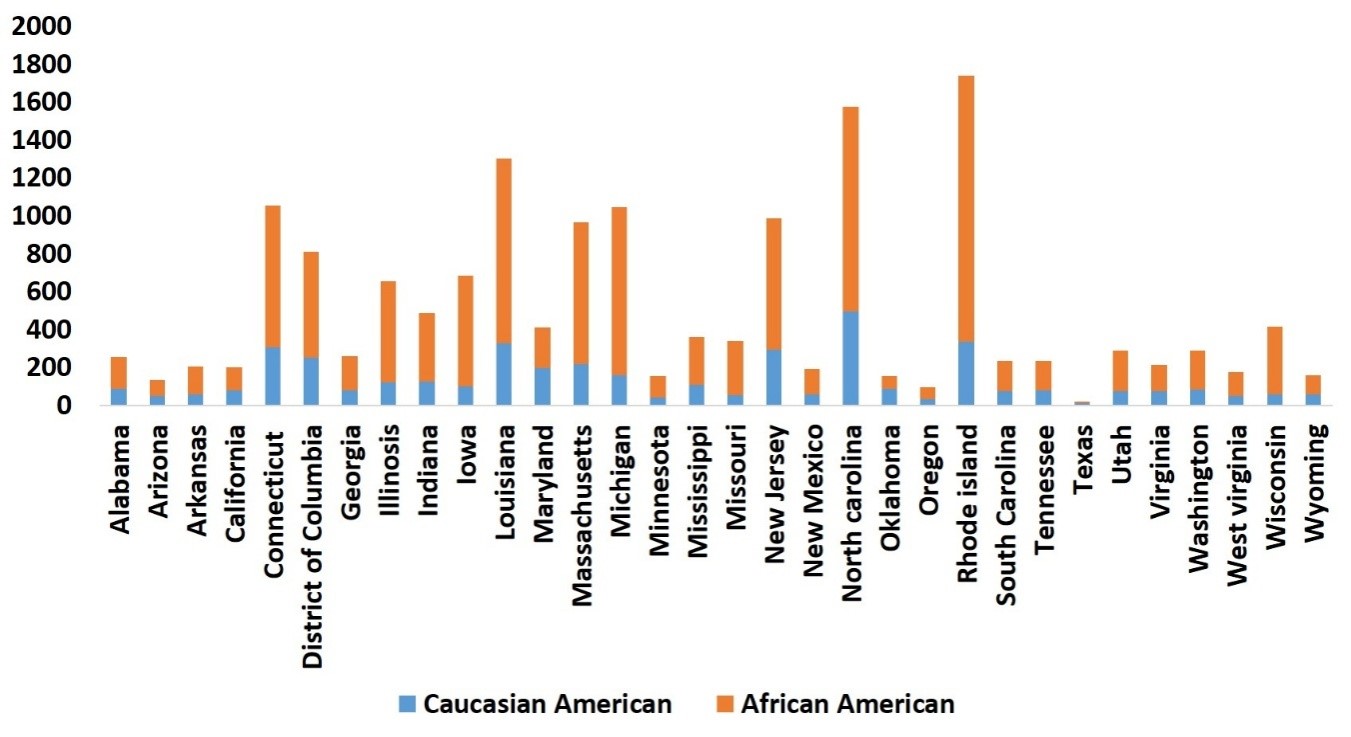
Figure 3B: COVID19 case rate as of April 24th2020 per 100,000 populations by race.
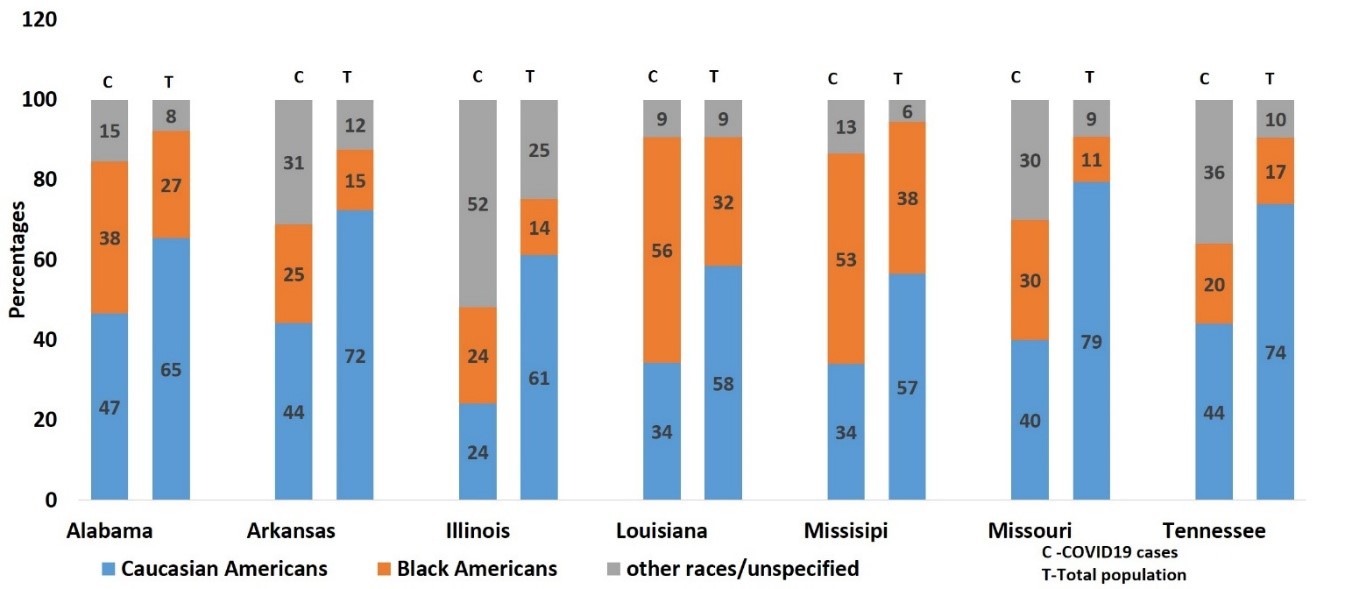
Figure 4: Percentage of COVID19 cases by race (N=95,156). as of April 24th 2020, and total US population(N=411,789,98) by race ,2017 in delta region of USA.
Citation: Kodidela S, Kumar A, Gerth K, Kumar S, Walker C (2020) Lessons Learned from Health Disparities Among African Americans in The HIV Epidemic: What to Expect for COVID-19 and Potential Approaches to Mitigate Health Disparity. Emerg Infect Dis Diag J: EIDDJ-100021