Annals of Medical & Surgical Case Reports
(ISSN 2652-4414)
Case Report
Anesthetic Management of a Patient with First Arch Syndrome and Acute Valvular Infective Endocarditis Presenting for Full Mouth Rehabilitation
Chan YM1, Leech J2, Maposa D2, Levesque V2 and Hierlmeier BJ2*
1Perioperative Services of Mississippi, Jackson, Mississippi, USA
2Department of Anesthesiology, University of Mississippi Medical Center, USA
Corresponding author: Bryan Hierlmeier, Department of Anesthesiology, University of Mississippi Medical Center, USA.
Citation: Chan YM, Leech J, Maposa D, Levesque V, Hierlmeier BJ (2020)Anesthetic Management of a Patient with First Arch Syndrome and Acute Valvular Infective Endocarditis Presenting for Full Mouth Rehabilitation. Ann Med & Surg Case Rep: AMSCR-100051
Received date: 27 March, 2020; Accepted date: 30 March, 2020; Published: 06 April, 2020
Abstract
Clinical effects of multivalvular disease is complex making anesthetic management of noncardiac surgery a challenge. Generalizations can be made about hemodynamic goals, but pathophysiology is dynamic and unique to each patient and must be treated as such.The combination of aortic regurgitation (AR) and mitral regurgitation (MR) is usually poorly tolerated because the double-valve regurgitation tends to exacerbate left ventricular volume overload.When these cases are complicated with craniofacial abnormalities, overall anesthetic challenge is amplified.
Keywords: Acute aortic valve insufficiency; Acute mitral valve insufficiency; Difficult airway;Endocarditis; First arch syndrome
Introduction
Patients with acute multivalvular regurgitations are critically ill and hemodynamically compromised.One cause of non-ischemic valvular regurgitation is infective endocarditis (IE).Left-sided, double valve IE is rare but not unheard of [1]. In patients with native valve disease, the prevalence of multiple valve involvement has been reported as high as 20.2% [2].Urgent/emergent surgical treatment is often required either via valve repair or valve replacement [3,4].Prior to valve replacement, perioperative dental care is commonly requested in an attempt to minimize the risk for late development of prosthetic valve endocarditis.
The anesthetic management for non-cardiac surgery in this patient population is complex.The risk of major adverse event associated with dental extraction prior to cardiac surgery is as high as 8% [5]. Pre-existing co-morbidities further complicate an already challenging anesthetic particularly when a difficult airway is anticipated.Despite the prevalence of multivalvular disease and its hemodynamic impact on anesthetic management, there is a paucity of discussion in literature to guide perioperative care.We aim to contribute to this body of work by presenting our unusual patient.
Case description
A 22-year-old Caucasian male presented to the operating room for full mouth rehabilitation in preparation for early simultaneous aortic and mitral valve replacement.Past medical history included first arch syndrome, cognitive delay, attention deficit and hyperactivity disorder and hyperthyroidism.Home medications included clonidine, divalproex, olanzapine, pantoprazole and ondansetron.
He was transferred to our facility in severe sepsis and diagnosed with bacterial endocarditis.He was admitted to the ICU with initiation of antibiotics.Physical exam was notable for tachypnea, tachycardia, poor dentition, petechial rash, splinter hemorrhages, and arthritis along with a grade III murmur heard best at lower left sternal border.Relevant lab test results included platelet count of 82,000/mL and acute renal insufficiency (BUN of 43mg x dL-1 and creatinine of 1.5mg x dL-1).
Over the course of 4 days before the dental procedure, serial echocardiography and CT scans showed progressive worsening of endocarditis and bilateral pleural effusion.Two-days prior to dental restoration, TTE exam found normal left ventricular size and systolic function with estimated LV ejection fraction of 40%, estimated RV systolic pressure of 40mmHg, severe mitral regurgitation from a flail posterior leaflet (Figure1), and large circumferential pericardial effusion with early tamponade physiology (Figure 2).A pericardial drain evacuated 800mL of serosanguineous fluid that day upon insertion.The night before surgery, the pericardial drain put out another 1000mL.He was admitted to the intensive care unit (ICU) with on-and-off norepinephrine infusion prior to coming to the operating room.
Preoperatively, the patient was assessed in the ICU where he had been awake and alert and did not complain of distress; however, it was apparent that he is dyspneic when speaking.According to his mother, he received sedation on multiple occasions for dental restoration, but as far as they know, he had not received general anesthesia previously.Mother recalled past anesthetic providers voicing concerns about his airway.His parents, who claimed no family history of anesthetic complications, provided his history.
On airway exam, it was clear that he suffered from craniofacial anomalies.Patient presented with bilateral micrognathia, maxillary hypoplasia and external ear deformities as seen in Figure 3.His mouth opening was limited without ability to protrude his mandible.His palate was higharched with a Mallampati class III airway.There was no limitation on neck range of motion.
After informed consent was obtained, the patient was transported to the operating room (OR).Standard ASA monitors were applied.Bilateral chest tubes were placed on suction.Patient had an in situ 20-gauge radial arterial line.Using aerosolized 4% lidocaine, patient’s airway was anesthetized. Then, he was carefully sedated with midazolam, fentanyl, and ketamine in small incremental doses and fiber optically intubated through the mouth using a size 7.5 endotracheal tube over a pediatric flexible bronchoscope.Intubation proceeded with ease.His airway was secured on the first attempt with stable vital signs throughout procedure (heart rate of 143 beats per minute, blood pressure of 130/55mmHg and oxygen saturation in the low 90%).Total sedative doses for intubation included midazolam 1mg, fentanyl 25mcg, and ketamine 20mg.
However, after intubation, it quickly became apparent that patient did not tolerate 0.4% expiratory level of sevoflurane and positive pressure ventilation.Heart rate stayed in the 130’s to 140’s, but the systolic, diastolic, mean arterial blood pressures and pulse pressure began to fluctuate wildly beat-to-beat.Some blood pressure numbers registered include 109/10mmHg and 55/26mmHg all of which pointed to impending hemodynamic collapse.Phenylephrine 50mcg was bloused, sevoflurane discontinued, and the patient was able to breath spontaneously-after which, he recovered immediately.It was decided that anesthesia will be maintained with a low-dose propofol infusion at 25mcg x kg x min-1 with carefully titrated boluses of midazolam and ketamine for the remainder of the case.The Dentists were able to supplement analgesia with local anesthetics.Hemodynamics were very stable for the remaining operative experience.
At the end of the dental extractions, patient was intubated and transported in stable condition back to the ICU. On the day following dental procedure, TTE exam showed additional severe aortic regurgitation with vegetation (Figure 4), posterior pericardial effusion with fibrinous strands throughout and an echogenic mass within the pericardium likely representing a new vegetation.Five days later he underwent successful aortic and mitral valve replacement.
Discussion
Our patient presented with multiple active physiologic derangements.He suffered from sepsis, severe acute aortic and mitral valve regurgitation, pericardial and bilateral pleural effusions all of which interacted to produce complex and sometimes conflicting hemodynamic goals.His physiology was dynamic, changing from moment to moment, and difficult to predict.His cardiac output and propensity for forward flow is also likely to be exquisitely sensitive to changes in preload, afterload, contractility and other variables like intrathoracic pressure.The fact that he was alive and appeared to be in no distress, despite critical illness, speaks to the vitality of youth and the aegis of prior good health and we were wary to disrupt his delicately compensated physiology.
Ideally, perioperative management would be guided by echocardiography.Direct visualization of the cardiac chambers negates any guesswork on diagnosing the cause(s) of hypotension.Unfortunately, transesophageal echocardiography (TEE) hinders dental extraction, and transthoracic echocardiography (TTE) was unavailable to us during the case.
First arch syndrome, which was first described in 1889, occurs roughly in 1:50,000 live births.The most prominent abnormalities are malar and maxillary hypoplasia, cleft palate, and the absence of the zygomatic arch.Since it is a developmental defect, the presentation and airway implication are on a spectrum [6,7]. Examples of first arch syndrome include Treacher Collins and Pierre Robin syndrome – classic causes of difficult intubation.Since our patient had never been intubated and was unlikely to tolerate prolonged hypoxemia/hypercarbia, we decided to proceed with fiberoptic intubation.
We were uncertain how this patient’s physiology would respond to some events not uncommonly anticipated with awake fiberoptic intubation – coughing, Valsalva maneuver, hypoxia and hypercarbia (and their effects on pulmonary vascular resistance and catecholamine release), and the need for positive pressure ventilation (PPV).While PPV can be lifesaving, it certainly has impact on left ventricular (LV) and right ventricular (RV) physiology that may or may not be favorable depending on other parameters [8]. For these reasons, we aimed to secure the airway as smoothly and comfortably as possible.We chose to sedate our patient, dosing in small mindful increments, since patient and family gave clear history of having tolerated sedation in the past for dental procedures.
As our case illustrates, pre-induction arterial line promotes rapid recognition and intervention when hemodynamic decompensation is imminent.On a beat-to-beat basis, arterial waveform graphically illustrates the dynamic interplay between the stroke volume, the speed of the volume ejected, the distensibility of vascular tree, and the flow rate of ejected blood from central arterial compartment to the periphery [9].
Although generalizations can be made about perioperative management goals, we cannot overemphasize the importance of treating each patient’s pathophysiology as unique and dynamic, one that can shift in an instant.For example, a common recommendation for management of mitral regurgitation is to keep heart rate in the high normal range (80 to 100 beats per minute),[10] a good starting point, yet our patient had been in sinus tachycardia in the 130-140’s, stable and in no apparent distress for days.There may be value in considering baseline vital sign trends that the patient has been tolerating or even “preferring” over the hospital course.In a case series of 9 patients with IE leading to AR with or without MR, the mean heart rate was 108 +/- 15 beats per minute or ranged between 93 to 124 beats per minute [11].Of these nine patients, the highest pulmonary artery wedge pressure recorded was in the two patients with both severe AR and MR.
The clinical significance of multivalvular disease depends on the valves involved, the type and severity of involvement, the acuity of disease, the loading conditions and ventricular compensation [12].In combination, acute AR and MR is poorly tolerated as the volume overload tends to be extreme [12].Forward flow stroke volume is predominantly dependent on systemic vascular resistance (SVR) followed by myocardial contractility.It was not a surprise then, sevoflurane, even in low dose, depressed myocardial contractility enough to be perilous.
According to an article published by the American College of Cardiologists, it was noted that positive pressure ventilation improved forward flow in patients with left heart failure and severe MR because extrinsic compression of the aortic arch activates baroreceptor reflex thus decreasing afterload.Furthermore, LV diameter would decrease, and LV wall tension would increase which implies improved contractility [8]. However, it is unclear how positive pressure ventilation would impact flow in the setting of both acute AR and acute MR.Our patient responded poorly to positive pressure ventilation.It is possible that despite the pericardial drain, there was still enough pericardial fluid around the heart to create a tamponade effect making the heart pre-load dependent and worsened by positive pressure ventilation.
Finally, we would like to suggest that the successful anesthesia management of this case was dependent on a threshold concept in anesthesiology.According to Barry and Little wood, “threshold concepts can be at least superficially defined as ways of thinking and practicing that are so fundamental to a discipline that they are required for expertise, that their mastery results in a fundamental transformation of self and one’s relationship with their discipline, and that they are associated with a rupture from prior ways of knowing and doing”[13].Perioperative management of complex physiology requires a responsive anesthetic.The exact physiology at any moment is ambiguous, making it difficult to predict which cardiovascular determinant is dominant and to what degree its impact.Yet we must try constantly to gauge the current status, integrate that information with our knowledge of pathophysiology, span and project into the future.Only through iterations of hypothesis-making and testing can we hope to grapple with such complexity.
Conclusion
Anesthetic management for non-cardiac surgery of patient with acute multivalvular regurgitation, sepsis and pericardial effusion is challenging particularly when echocardiography is unavailable or infeasible.Hemodynamic goals may be conflicting, dynamic and hard to predict.The impact of positive pressure ventilation and effects of difficult airway further contribute to complexity.Vigilance and thorough understanding of pathophysiology and pharmacology helps supports individualized care of these critically ill patients through the perioperative period.
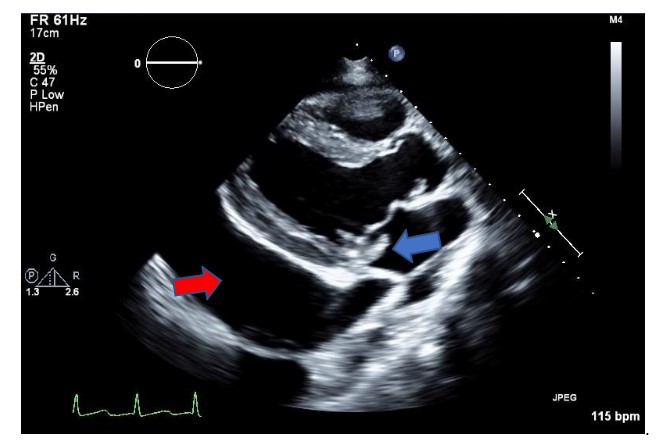
Figure 1: Transthoracic echo parasternal long axis view of the left ventricular showing a posterior mitral leaflet flail segment (blue arrow) and a large pericardial effusion (red arrow).
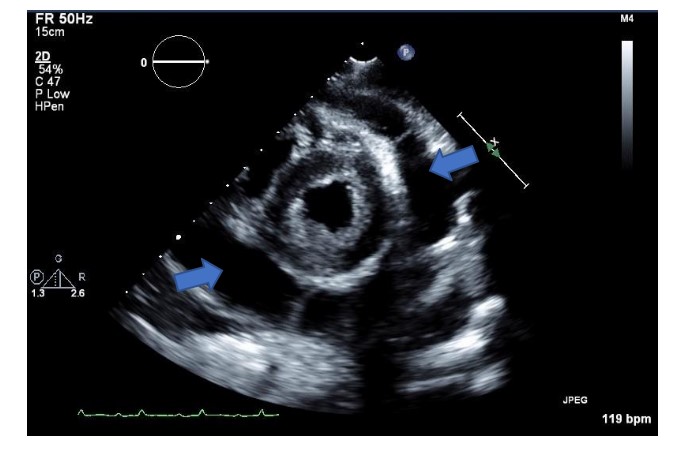
Figure 2: Transthoracic parasternal short axis view of the left ventricle showing larger pericardial effusion (blue arrows).
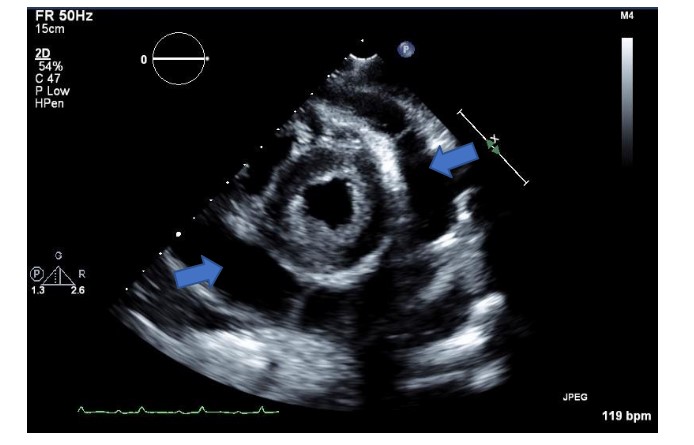
Figure 3: AP and lateral images of a patient with First Arch Syndrome showing micrognathia, maxillary hypoplasia and external ear deformities along with high-arched palate.
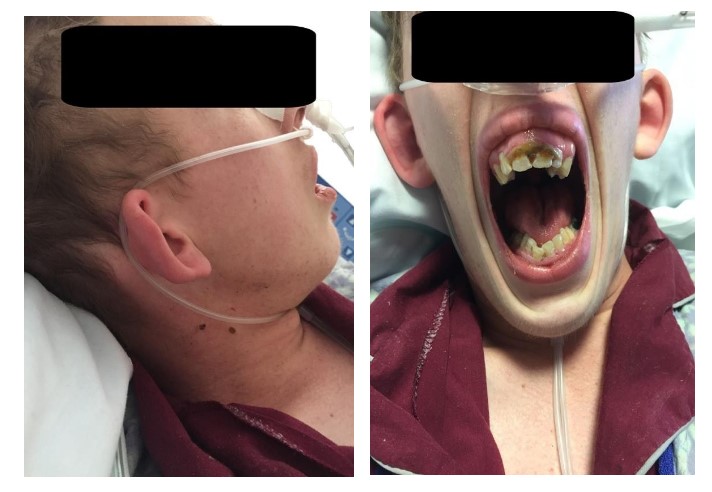
Figure 4: Transthoracic echo parasternal long axis view of the left ventricle showing a posterior mitral leaflet flail segment (blue arrow) and now a large vegetation of the aortic valve (red arrow).
Citation: Chan YM, Leech J, Maposa D, Levesque V, Hierlmeier BJ (2020)Anesthetic Management of a Patient with First Arch Syndrome and Acute Valvular Infective Endocarditis Presenting for Full Mouth Rehabilitation. Ann Med & Surg Case Rep: AMSCR-100051