Emergency Medicine and Trauma Care Journal
(ISSN 2652-4422)
Review Article
Obesity-Pathogenes Development Energy Imbalance
Mukhamejanov E*, Kairanbayeva G and Nysanova B
Professor, Chief Researcher in the Pharmacology and toxycology laboratory, Kazakhstan
*Corresponding author: Emil Mukhamejanov, Professor, Chief Researcher in the Pharmacology and toxycology laboratory, Kazakhstan.
Citation: Mukhamejanov E, Kairanbayeva G, Nysanova B (2020) Obesity-Pathogenes Development Energy Imbalancem.Emerg Med Trauma. EMTCJ-100031
Received date: 23 March, 2020; Accepted date: 26 March, 2020; Publication date:06 April, 2020
Abstract
The mechanism of obesity is an imbalance between the amount of food compounds and their use in the processes of life. Therefore, the mechanism of obesity development should take into account both all stages of energy formation and ways of its use. Usually in matters of prevention and treatment of obesity pay attention at the number of calories coming from food and their use for physical activity, so all technologies are based on reducing the amount of calories and increasing their spending on physical activity. In this report, the main attention in the pathogenesis of obesity is paid to the assessment of the stages of activation of energy-dependent processes, in particular the stages of protein and glucose synthesis. This approach allows a new look at the pathogenesis of obesity and opens up prospects for the prevention and treatment of this disease.
Keywords:Energy-dependent processes;Obesity; Pathogenesis;Prevention; Treatment
Introduction
Obesity is a global epidemic with more than 35% of the world population (2,100 million people) being estimated as either overweight or obese according to body mass index (BMI) [1]. Obesity is associated with a large number of health problems including dyslipidemias, cardiovascular diseases (CVD), type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver disease (NAFLD), and some types of cancer, with important economic and social costs [2]. Systematic analyses have revealed that obesity and overweight caused 3.4 million deaths in 2010[3].
The mechanism of the development of obesity is a mismatch between the number of incoming calories and the amount of their utilization.It was this simplicity of view that brought the whole problem to a standstill. Therefore, all technologies for the prevention and treatment of obesity are aimed at reducing the amount of calories and increasing their impact on physical activity. Indeed, when reducing the diet and increasing physical activity, there is a decrease in body weight, but often this is a temporary phenomenon and very often there is the development of various functional disorders. Man is a weak creature and he wants to eat and lie on the couch, so the number of obese persons is constantly increasing and humanity is losing the battle with obesity [4].
Energy we mainly spend on physical activity, protein synthesis and heat production.The literature mainly deals with the use of energy for physical activity and heat production, but little attention is paid to the issues of energy production and its use for anabolic processes, in particular the process of protein synthesis. In this article we will focus on the issues of energy production and its use on anabolic processes (protein and glucose synthesis).With food, we get all the necessary building, energy and regulatory compounds, so nutrition is the main factor of the body's life and in relation to proper nutrition, scientific forums are constantly held, many monographs have been written, but there are still many unclear questions.
The rate of any chemical reaction depends on the concentration of the substance (C), temperature (t) and pressure (p). But on such principles can live only unicellular organisms, in particular bacteria, vital activity which can increase when the amount of food increases or simply stop (suspended animation) in its absence. In highly organized organisms and in humans, metabolic rates can change dramatically [5]. Therefore, at a certain stage of their development, a hormonal system was created (hormones can increase the rate of chemical reactions by tens or even hundreds of times). But this was not enough, so at the next stage of development, the nervous system was created (mediators can change the speed of metabolic processes hundreds or even thousands of times). These positions are represented by us in the form of a pyramid of regulation(Figure 1).
At the bottom of the pyramid are the main metabolic processes or exchange of macronutrients (proteins, fats and carbohydrates) and micronutrients (vitamins and trace elements). Under load, oxygen consumption can increase 200 times [6]. Such an increase in the rate of metabolic processes is not possible due to chemical principles; specific sensors (regulators) are required. Therefore, in order to maintain the processes of vital activity, the body needs not only an adequate supply of all food compounds, but also that they are effectively included in the relevant metabolic processes, which is not taken into account when developing the principles of nutrition.
The long-term consumption of unbalanced diets (high content of calories, fat, fructose and high omega-6/omega-3 fatty acid ratio), coupled with the adoption of a sedentary lifestyle, contributes to the development of obesity and associated complications [7]. Also, it is now recognized that interactions of genetic and epigenetic signatures with environmental factors (dietary intake or physical activity) play an important role in determining individual phenotypes [8]. Recent advances in genomic sequencing and large cohort studies are enabling clarification of the involvement and the interplay of these factors in chronic disorders including obesity, which open a new field to customize intervention strategies [9].
The incidence of obesity and related diseases is rapidly increasing, more than 100-fold over the last three decades without showing the slowdown [10]. Obesity represents a symptom of about 40 monogenic diseases or chromosomal abnormalities [11].However, as genomics affects food assimilation [12] and Back [13], food components affect gene expression and, therefore, metabolomics. Food compounds cause the expression of genes responsible for the dissimilation and assimilation of food compounds and, in the end, can cause the consolidation of these aspects in the offspring through natural selection.But this is a slow process and it cannot explain the high rate of increase in the number of obese persons.
The lifestyle of modern man has changed dramatically and the available genotype sometimes does not correspond to the activity of some metabolic conveyors. Therefore, it will be more appropriate not to adjust the nutrition for the genotype, but to use the nutrition corresponding to the nature of modern human activity.In this regard, it is necessary to understand the features between anabolic processes and their energy supply.
It is clear that the energy supply of life processes plays a paramount role. In hygiene, energy expenditure is usually associated with the amount of motor activity. However, metabolic energy expenditure can often exceed physical. Thus, 3 ATP is spent on the formation of a peptide bond or a compound of two amino acids. The average protein contains about 100 peptide bonds; thousands of proteins are synthesized per day, so protein synthesis is the most energy-consuming process in the cell. Previously, we proposed a model of the relationship between anabolic processes and their energy supply (Figure 2).
In the center of the model is protein metabolism. It coordinates the metabolism of carbohydrates and lipids. In the absorptive period after a meal (a state of excess calories-the image of Sanchez punch), the energy produced by glucose catabolism is used on the anabolic process of protein synthesis. This stage determines the relationship between the metabolism of carbohydrates and proteins at the level of formation and utilization of ATP energy. If there are not enough carbohydrates in the diet, the value of ATP production decreases, which leads to a decrease in the rate of protein synthesis. Due to the decrease in the inclusion of amino acids in proteins, their accumulation occurs, and manifestations of hyperaminoacidemia develop, which is noted when using high-protein diets.
On the contrary, with a deficiency in the diet of protein, the amount of protein synthesis decreases or the use of ATP energy decreases. This leads to an increase in the ATP/ADP ratio, which leads to inhibition of glycolysis. First of all, this applies to muscle tissue, which is about half of the skinny body weight.Muscle mass is controlled by complex interactions of multiple factors; however, the dynamic balance between protein synthesis and breakdown is a major determinant of it [14]. There is a decrease in the utilization of glucose by the muscle, which leads to an increase in its concentration in the blood (hyperglycemia) and activates the process of "dumping" the carbon skeleton of glucose into fats and increasing the level of lipids in the blood (hyperlipidemia) and increasing their deposition in adipocytes.
For each individual, the ratio between carbohydrates and proteins will depend on his metabolic characteristics, the nature of work, environmental factors and the time of year, which allows you to choose for each person its ratio, which will not lead to the development of functional disorders. This ratio between carbohydrates and proteins is easily determined by simple biochemical screening by giving a Breakfast with a known ratio between macronutrients. If after taking Breakfast there are manifestations of hyperaminoacidemia, it should be increased in the diet of carbohydrates, and with the manifestations of glycemia and lipidemia, reduce the proportion of carbohydrates. This is a kind of principle of development of personalized nutrition, when nutrition is adjusted not to the genotype, but to all factors and, in particular, the genotype.
Since in the diet therapy of obese persons, high-protein diets are mainly used, which is often a factor in the development of hyperaminoacidemia and deterioration of human health. Reduce the manifestations of hyperaminoacidemia can not only by reducing the amount of protein in the diet, but also through the use of various anabolic technologies. High effect on increasing the rate of protein synthesis have resistant exercise [15], anabolic amino acids, especially leucine[16], which activates protein synthesis [17].
In the post-absorptive period or before the subsequent food intake (state of energy deficiency) includes energy need process endogenous synthase glucose (gluconeogenesis). When glucose is oxidized to pyruvic acid (pyruvate), 2 ATP molecules are released, and in the reverse synthesis of glucose from pyruvate, 6 ATP is expended or in the energy aspect, we additionally expend 4 ATP. Overwork in alanine for glucose synthesis is already spent 10 ATP, so the additional 4 ATP is spent on the formation of urea. At the time, we used in the diet of obese children taking L-alanine in an amount of 5 g/day, which significantly increased the effectiveness of diet therapy (unpublished data).
The more efficiently glucose is oxidized, the less its carbon skeleton will be dumped into fats. Therefore, let's analyze the process of glucose oxidation and the factors affecting this process. The main consumer of glucose under the influence of insulin are muscles [18]. Therefore, the insulin cascade is given special attention in the pathogenesis of insulin resistance - IR [19]. It is believed that insulin through its receptor promotes the flow of glucose into the muscle cell for which it must activate the enzyme hexokinase, but biochemical confirmation of this fact is not received.Glycolysis proceeds with the expenditure of two ATP molecules for the phosphorylation of glucose and fructose-6-phosphate [20]. The cell will not be wasting energy, i.e. these are important steps in glucose metabolism and must be tightly controlled in the cell. Therefore, to pass glucose into the cell, appropriate “block posts” are put, which control the cell's need for glucose. Hexokinase is involved in glucose phosphorylation, so the first control is at the level of its activity and, on the contrary, the accumulation of its reaction product (glucose-6-phosphate) on the basis of feedbackinhibits the activity of the enzyme [21]. The second post block is the regulation of glycolysis at the second stage of phosphorylation [22].
Phosphorylation of fructose-6-phosphate into fructose-1,6-diphosphate enabled activation of the conversion pathway of the six carbon compound to three carbon (trioses), which are further oxidized to pyruvate, further conversion of which can go in several ways:
With the participation of Lactate dehydrogenase, pyruvate is converted in to lactic acid (lactate); with the participation of Alanine aminotransferase, pyruvate is converted to the amino acid alanine; with the participation of Pyruvate carboxylase, pyruvate is converted to oxaloacetate; with the participation of Pyruvate dehydrogenase, pyruvate is converted to Acetyl-CoA(Figure 3).
Therefore, a violation of the metabolism of pyruvate leads to the development of a number of diseases [24]. It is known that all compounds lying at the crossroads of metabolic pathways in the body should be maintained at the homeostatic level, therefore the process of its formation is inhibited by the feedback principle, i.e. glycolysis (“pyruvate block”) is inhibited and, accordingly, glucose utilization decreases or IR develops.
In the body at the same time work all the ways of turning pyruvate. When recovering pyruvate to lactate, the recovered equivalents formed during glycolysis are used. This is not a very economical way of glucose oxidation, since it only releases 7% of the energy of chemical bonds of glucose, but this is an important stage of life preservation, as the NAD/NAD.H2 factor is maintained (an important aspect of life preservation), but this leads to a decrease in the substrate (pyruvate) for other metabolic processes.
Under anaerobic conditions, pyruvate can also turn into alanine during transamination. Branched chain amino acids (leucine, valine, isoleucine) act as substrates for the supplier of amino groups for transamination; therefore, the intake of these amino acids leads to an increase in the utilization of pyruvate and is the prevention of diabetes [25]. In these cases, branched-chain amino acids, and especially leucine, will act as an informational molecule to enhance protein synthesis during the transcription and translation stages [26].
Under aerobic conditions, pyruvate can add carbon (carboxylate to oxaloacetate) or release carbon (decarboxylate to acetyl CoA). Vitamin B1, magnesium, lipoic acid are involved as cofactors in carboxylase and pyruvate dehydrogenase activities; therefore, their deficiency impairs the activity of these enzymes, decreases the amount of pyruvate utilization, and develops a pyruvate block; therefore, there are many data on the deficiency of these compounds in people with diabetes and obesity [27-29].
If oxaloacetate and acetyl-CoA can enter the mitochondria, but the structural and functional activity of mitochondria is impaired or the possibility of including oxaloacetate and acetyl-CoA is reduced in the tricarboxylic acid cycle (TCA), the principle of feedback again turns on and the process of pyruvate conversion is automatically broken in oxaloacetate and acetyl CoA. Indeed, in patients with diabetes, structural changes in mitochondria are detected [30]. Reducing the inclusion of pyruvate in oxaloacetate and acetyl-CoA will lead to the restoration of pyruvate in lactate, so the level of lactate in patients with diabetes and obesity is increased [31]. From this point of view, when the process of glucose oxidation is disturbed, the accumulation of its exchange intermediators occurs, which, by the principle of feedback, inhibit the entry of glucose into the cell or manifestations of IR are detected.
Acetyl-CoA can be formed from all macronutrients (proteins, fats and carbohydrates)and therefore it is believed that there is a relationship between the exchange of macronutriets. However, in metabolic terms, the level of acetyl-CoA should be maintained at homeostatic level, so there is more competition between macronutrients for the supply of acetyl-CoA. Most clearly, this competition is manifested between carbohydrates and fats of food. This competition is under hormonal control. So in the absorptive period there is a secretion of the hormone insulin, which inhibits the oxidation of lipids and thereby reduces the possibility of formation of acetyl-CoA from fats, so the main supplier of acetyl-C0A in this period are carbohydrates. But with obesity, IR develops and fats can already compete with carbohydrates for the supply of acetyl-CoA, which leads to a decrease in their utilization or the Randle cycle glucose-fatty acids is activated [32]. This leads to a decrease in the utilization of glucose by tissues, its translation into fats and deposition in adipocytes.
Therefore, reducing IR is a targeted principle of prevention and treatment of obesity. As already discussed earlier in the pathogenesis of the development of IR, it is important to reduce the amount of glucose oxidation at different stages of glycolysis, so in the prevention and treatment of obesity, important importance should be given to technologies to eliminate obstacles to glucose oxidation, which have already been considered earlier.
The main amount of acetyl-CoA is formed during lipolysis of fats. However, the oxidation of fats to acetyl-CoA occurs in the cytoplasm of cells, and its oxidation is carried out in the mitochondria. However, the mitochondrial membrane is impervious to acetyl-CoA and a Transporter is required. Carnitine acts as such a carrier (Figure 4).
First, in the mitochondrial membrane, the acetyl group is transferred to carnitine to form acyl-carnitine, and then the acyl group on the other side of the membrane is transferred to CoA and acetyl-CoA is formed on the other side of the membrane. Therefore, with carnitine deficiency, the transfer of acetyl-CoA from the cytoplasm to the mitochondria is disrupted, which leads to inhibition of lipid oxidation. Since the maintenance of acetyl-CoA homeostasis is an important aspect of the regulation of metabolic processes, when the oxidation of fats to acetyl-CoA increases, two molecules of acetyl-CoA condense into aceto-acetate and then its transformation into oxybutyrate and acetone or ketosis develops, which has a toxic effect on brain activity [33]. Manifestations of ketosis are particularly well identified in diabetes and fasting, when the relationship between energy production and its utilization for gluconeogenesis is disrupted.
The concentration of carnitine decreases in obesity [34], which may be the basis for the destruction of lipid oxidation. Indeed, carnitine contributes to the reduction of the acetyl-CoA in citosole level, which leads to an improvement in the oxidation of fat marked by the reduction [35]. Carnitine helps to eliminate dysregulation of fat oxidation in obesity [36].
In mitochondria, acetyl-CoA dehydrogenation occurs and the resulting reduced equivalents (electrons) enter the biological oxidation cycle and ATP is produced. At the first stage of phosphorylation, coenzyme Q10 is involved, the level of which decreases with age [37], which leads to inhibition of the oxidation rate of acetyl-CoA and, respectively, lipids. CoQ10H2 content in adipose tissue gradually decreased with the development of obesity in both mice and humans, and that CoQ10H2 synthesis-related enzymes were upregulated as a compensatory measure [38]. In the experimental study [39], it was shown that coenzyme Q10 contributed to the reduction of visceral fat. Therefore, in the mechanism of obesity plays an important role violation of the processes of aerobic oxidation of organic compounds.
Conclusion. In the mechanism of development of energy imbalance in obesity of great importance, in addition to excessive intake of calories and their expenditure on physical activity, plays the effectiveness of metabolic processes. With a decrease in the efficiency of energy production at the anaerobic and aerobic stages and deterioration in the efficiency of energy utilization for energy-dependent processes, in particular for the synthesis of protein and glucose, there is an increase in the deposition of organic compounds in the form of fats. Technologies that improve the process of energy production and increase the efficiency of anabolic processes (protein and glucose synthesis), will be an important method of prevention and treatment of obesity.
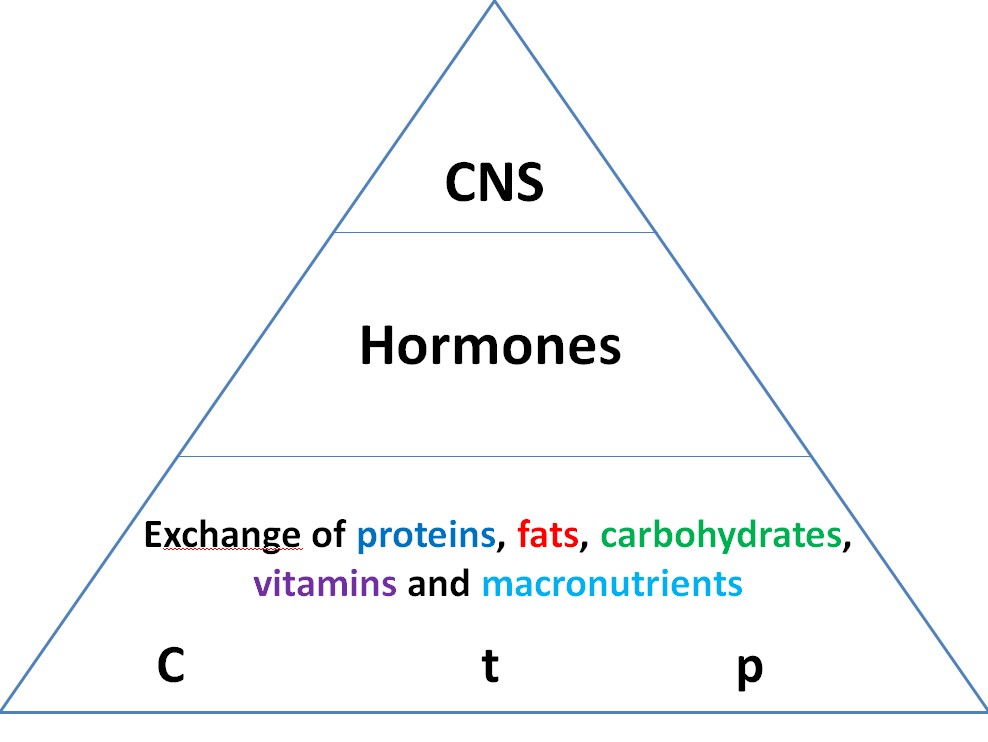
Figure 1:Regulation of metabolic processes in the form of a pyramid.

Figure 2:Model of the relationship between protein, fat and carbohydrate metabolism to maintain glucose homeostasis.
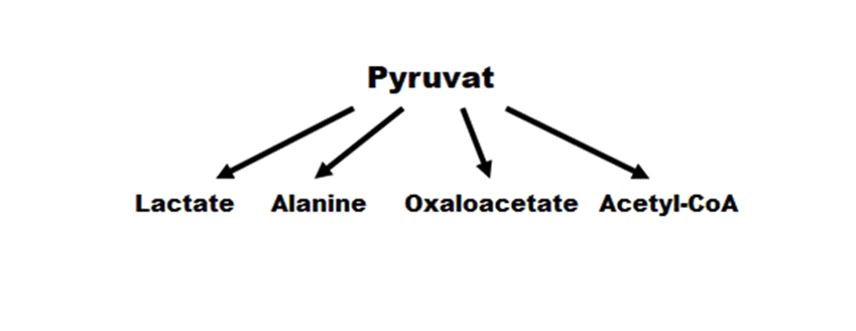
Figure 3: Ways to make pyruvate.
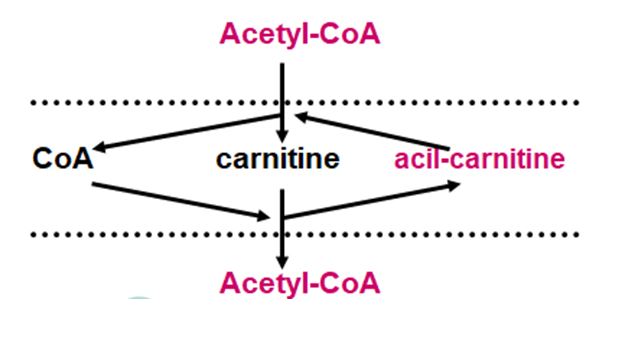
Figure 4:Scheme of the involvement of carnitine in the transport of acetyl-CoA from the cytoplasm to the mitochondria.
Citation: Mukhamejanov E, Kairanbayeva G, Nysanova B (2020) Obesity-Pathogenes Development Energy Imbalancem.Emerg Med Trauma. EMTCJ-100031