Journal of Diabetes Management and Metabolism
(ISSN 2652-4430)
Short Review
Diagnosis and Management of Bone Fragility in Diabetes: An Emerging Challenge
Ferrari SL1*, Abrahamsen B2, Napoli N3, Akesson K4, Chandran M5, Eastell R6, El-Hajj Fuleihan G7, Josse R8, Kendler DL9, Kraenzlin M10, Suzuki A11, Pierroz DD12, Schwartz AV13, Leslie WD14, on behalf of the Bone and Diabetes Working Group of IOF*
Bone and Diabetes Working Group of IOF*
SL Ferrari, B Abrahamsen, K Akesson, MSM Ardawi, M Chandran, C Cooper, R Eastell, G El-Hajj Fuleihan, R Josse, DL Kendler, M Kraenzlin, WD Leslie, A Mithal, N Napoli, A Suzuki, AV Schwartz
1Faculty of Medicine, Geneva University Hospital, Geneva, Switzerland
2Department of Medicine, Holbaek Hospital, Holbaek, Denmark and OPEN, Institute of Clinical Research, University of Southern Denmark, Odense, Denmark
3Department of Medicine, Università Campus Bio-Medico di Roma, Roma, Italy; Division of Bone and Mineral Diseases, Washington University in St Louis, St Louis, MO, USA
4Department of Clinical Sciences, Clinical and Molecular Osteoporosis Unit, Lund University, Malmö, Sweden
5Osteoporosis and Bone Metabolism Unit, Department of Endocrinology, Singapore General Hospital, Singapore
6Academic Unit of Bone Metabolism, Mellanby Centre for Bone Research, University of Sheffield, Sheffield, UK
7Department of Internal Medicine, American University of Beirut Medical Center, Riad El Solh, Beirut, Lebanon
8Department of Medicine and University of Toronto, and Division of Endocrinology and Metabolism, St. Michael's Hospital, Toronto, Canada
9Department of Medicine, Division of Endocrinology, University of British Columbia, Vancouver, Canada
10Endonet, Endocrine Clinic and Laboratory, Basel, Switzerland
11Division of Endocrinology and Metabolism, Fujita Health University, Toyoake, Aichi, Japan
12International Osteoporosis Foundation, Nyon, Switzerland
13Department of Epidemiology and Biostatistics, University of California, San Francisco, CA, USA
14Department of Internal Medicine, University of Manitoba, Winnipeg, Manitoba, Canada
*Corresponding author: Serge L Ferrari, Department of Internal Medicine Specialties, Geneva University Hospital & Faculty of Medicine, Geneva 14, Switzerland, Email: serge.ferrari@unige.ch
Citation: Ferrari SL, Abrahamsen B, Napoli N, Akesson K, Chandran M, et al. (2020) Diagnosis and Management of Bone Fragility in Diabetes: An Emerging Challenge. J Diabetes Metab Manag: JDMM-10008
Received date: 24 January, 2020; Accepted date: 28 January, 2020; Published date: 04 Febraury, 2020
Fragility fractures as a complication of diabetes
Fragility fractures are increasingly recognized as a complication of both type 1 and type 2 diabetes [1]. Patients with type 1 diabetes, have increased risk of fractures throughout their life span, with hip fracture incidence occurring 10 to 15 years earlier compared to those without diabetes [2]. Meta-analyses published by Janhorbani [3] and Vestergaard [4] show a strong association and effect size for type 1 diabetes (RR 6.3 and 6.94 respectively) compared to type 2 diabetes (RR 1.7 and 1.38 respectively), in both men and women. Considering the increasing prevalence of diabetes and the fact it may also be associated with greater risk for injurious falls [5], fragility fractures increasingly appear as a serious, yet neglected complication of this disease.
Diabetes-related risk factors for fractures
Certain individuals with diabetes seem to be at greater risk of fracture than others. Evidence from recent studies has shown that longer duration and/or poor glycemic control could further increase fracture risk in diabetes [6]. In type 2 diabetes, age and duration of diabetes are clearly important [7-10]. A biphasic pattern has been proposed where fracture risk is decreased in newly diagnosed type 2 diabetes patients, which could be related to some protective effects of increased fat mass in these subjects, and only increases significantly after 5 years [11]. In analyses adjusted for baseline fracture risk using the Fracture Risk Assessment tool (FRAX®), only type 2 diabetes duration longer than 10 years was associated with a higher risk for major osteoporotic fractures while hip fracture risk was significantly increased for all durations [12].
Impact of diabetes medication on fracture risk
Observational studies reviewing different medications have often reported an increased fracture risk in patients taking insulin [13]. Patients receiving insulin and possibly insulin secretagogues are at higher risk of fracture through an indirect effect, related to hypoglycaemia-induced falls [14, 15]. Epidemiological data on diabetes patients taking sulphonylureas has revealed an increased risk of fractures [16]. Both in vitro studies and clinical trials have proven that treatment with rosiglitazone and pioglitazone, thiazolidinedione (TZD) class drugs, causes bone loss [17]. Current guidelines suggest avoiding TZD drugs in postmenopausal women or in men with other risk factors for bone fragility. However, clinical data confirms in vitro data that metformin has either a neutral or a possible beneficial effect on fractures, making this widely used medication a safe option regarding bone health [16].
The newer medications, incretin mimetics, dipeptidyl peptidase-4 (DPP4) inhibitors and glucagon-like peptide-1 (GLP-1) analogues, have a safe skeletal profile in type 2 diabetes [17]. Recent reports on sodium glucose cotransporter 2 (SGLT2) inhibitors have suggested that indirect activation of the FGF23-1,25 dihydroxyvitamin D-parathyroid hormone axis might contribute to adverse effects on bone health [18]. These have indicated decreased bone density and higher risk of peripheral upper and lower limb fractures in patients treated with canagliflozin [19] but not with empagliflozin [20]. Evidence suggest that empagliflozin did not increase the risk of bone fracture compared with placebo in a pooled analysis of >12,000 patients or compared with glimepiride in a 4-year head-to-head study [21]. The study of canagliflozin was a carefully conducted meta-analysis of RCT’s with adjudication of fracture events that found a modest increased fracture risk of 1.32 (1.00-1.74) [17]. The reasons for the discrepancy between the findings for canagliflozin and empagliflozin are not understood. More data are necessary to understand the effect of these new medications on bone health. Caution is recommended in prescribing SGLT2 inhibitors to those with higher fracture risk.
Bone mineral density and trabecular bone score
Most studies have shown that people with type 1 diabetes have lower bone mineral density (BMD) compared to healthy subjects [22]. In contrast, a 5 to 10% higher areal BMD is observed in type 2 diabetes patients in comparison to healthy subjects [4, 7, 23, 24], though there is significant heterogeneity between studies [24]. Lower BMD is a strong independent risk factor for fracture in diabetes patients [8]. However, for a given BMD T-score and age, the fracture risk was higher in type 2 diabetes patients compared to controls [25]. Thus BMD assessment remains useful to evaluate fracture risk in diabetes, but some adjustment of the T-score threshold for intervention is necessary see Figure 1.
*In diabetes, fracture risk at T-score <-2 equivalent for non-diabetes at T-score <-2.5 (see text) **depending on country-specific guidelines for therapies ***i.e. with TBS and/or “rheumatoid arthritis (RA)”-yes
+diabetes-specific CRFs: FRAX clinical factors, low BMD, recurrent falls, diabetes duration > 5 years, diabetes medications (insulin, TZDs, possibly SGLT2 inhibitors<), HbA1c >7%, microvascular complications (peripheral and autonomic neuropathy, retinopathy, nephropathy).
In certain countries humerus or pelvis fractures are also sufficient to initiate therapy; otherwise more than one non-vertebral non-hip fragility fracture could be required to initiate therapy; alternatively, a non-vertebral non-hip fragility fracture should prompt further exams to evaluate fracture risk.
In addition, spine trabecular bone score (TBS) tends to be lower among diabetes patients than controls. Moreover, within the type 2 diabetes group, TBS was better in those with good glycaemic control compared to those with poor glycemic control [26]. Hence TBS was found to be a BMD-independent predictor of fracture and predicted fractures equally well in those with (adjusted HR 1.27, 95% CI 1.10–1.46) and without diabetes (HR 1.31, 95% CI 1.24–1.38) [27, 28].
Bone architecture, quality, turnover and biochemical markers
Since reduced BMD alone does not fully explain bone fragility, especially in type 2 diabetes, alteration in “bone quality” is being investigated as a possible mechanism. With magnetic resonance imaging (MRI), Pritchard et al. measured larger holes in the trabecular network of type 2 diabetes compared to controls at baseline [29]. Using high resolution imaging at the distal radius and/or tibia, studies in postmenopausal women with or without diabetes suggest that there is a trend towards greater cortical porosity in type 2 diabetes compared to controls [30-32]. In a large U.S. cohort of older adults, type 2 diabetes was associated with greater cortical porosity [33]. In a recent study of 52 subjects with type 2 diabetes, of whom 25 had microvascular disease demonstrated, such cortical deficits were only present in patients with the microvascular complications [6].
The most important component of bone fragility in diabetes could be an impairment in the quality of collagen and its mineralization, through the accumulation of advanced glycation end products (AGE). Bone pentosidine levels, the most abundant AGE [34, 35], have been shown to be related to the strength of the human vertebra, independent of BMD [34]. Increased levels of serum pentosidine and AGEs were reported in type 2 diabetes compared to controls [36,37] and serum pentosidine was associated with greater risk of vertebral fracture in patients with type 2 diabetes [38]. In contrast the common bone turnover markers, such as CTX and P1NP, are usually not elevated in diabetes patients and are of little use in predicting fractures [39].
Bone fragility management in diabetic adults
Treatment of osteoporosis in patients with diabetes should be initiated when typical fragility fractures are present, since prior fracture predicts risk for future fracture as strongly in those with as without diabetes [8]. Otherwise, treatment should be considered at lower FRAX fracture probabilities (respectively higher BMD values) in diabetes patients than the general population, as both measurements underestimate fracture risk in this population, particularly those with type 2 diabetes [25, 40, 41].
Lifestyle intervention is always recommended in patients with diabetes and is the basis of any clinical guidelines. However, extreme weight loss without exercise to try and maintain muscle mass is associated with both muscle and bone loss that may increase the risk of bone fragility and sarcopenia [42]. Physical activity is highly recommended not only to prevent the progression of diabetes but also to increase BMD and to prevent the occurrence of sclerostin associated weight loss [43,44].
Tight glycemic control (HbA1c 6.5–6.9%) was associated with the lowest risk of fracture in a large cohort of elderly patients with diabetes [45]. However, both hypo- and hyper-glycemia are associated with increased fracture risk and falls [5], probably via different mechanisms.
With respect to anti-osteoporosis treatments, no randomized clinical trials have directly evaluated the antifracture efficacy of osteoporosis treatment in diabetes patients, particularly patients with BMD values and/or FRAX score above the standard intervention threshold. However, there is some evidence that osteoporosis drugs improve BMD equally in osteoporotic subjects with and without diabetes. Consequently, and in the absence of evidence against their use, bisphosphonates remain the first choice for osteoporosis treatment in diabetes patients (since data have not yet been published on the efficacy of denosumab in diabetes patients, it is too soon to provide recommendations about the use of this drug in those). However, the use and potential benefit of anti-resorptive drugs in patients with type 2 diabetes, typically characterized by relatively preserved BMD and/or normal to low bone turnover markers, and in whom bone fragility may mostly result from poor bone material properties, remains unproven and of potential concern. In this context, bone formation stimulating therapies such as teriparatide, and in the future abaloparatide or romosozumab, present a potentially interesting option.
Conclusion
Patients with diabetes are at increased risk of fragility fractures. Fracture risk is highest in type I diabetes, but evidence for risk assessment and treatment is scarce. Longitudinal studies have established that FRAX and BMD T-score predict fracture risk in those with diabetes, but in type 2 diabetes, both require adjustment of intervention thresholds for diabetes to avoid underestimation of risk. Currently available data suggest that a patient who has an indication for osteoporosis therapy based on criteria developed for non-diabetes patients should be treated with anti-osteoporosis drugs. In the absence of low BMD and/or typical fragility fractures though, these medications should be used with caution, as their effects on alterations in bone material quality remain to be thoroughly evaluated. Hence, our currently proposed algorithm should be considered as expert consensus which may change as more evidence emerges. A less stringent glucose control in elderly patients with type 2 diabetes to avoid hypoglycaemic events should be applied as well to falling risk, as recommended by the European Association for the Study of Diabetes (EASD) and the American Association of Diabetes (ADA) guidelines. The use of bone-safe medications, as well as the implementation of lifestyle intervention to prevent diabetes complications and sarcopenia, are also recommended. Meanwhile diabetes care/diabetes treatment centres provide an ideal opportunity for health care professionals to introduce evaluation and management of patients at high risk of fracture.
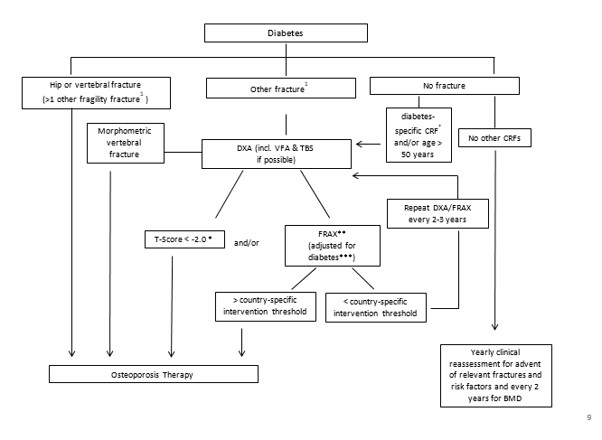
Figure 1: Fracture risk evaluation in patients with diabetes.
Citation: Ferrari SL, Abrahamsen B, Napoli N, Akesson K, Chandran M, et al. (2020) Diagnosis and Management of Bone Fragility in Diabetes: An Emerging Challenge. J Diabetes Metab Manag: JDMM-10008