José Luis da Silva Nunes*
Agronomist, Doctor in Plant Science, Technical of Development, Agribusiness Superintendence, Brazil
*Corresponding author: José Luis da Silva Nunes, Agronomist, Doctor in Plant Science, Technical of Development, Agribusiness Superintendence, Brazil. Tel: +555199834-8241.
Citation: Nunes JLS (2018) Anatomic and Morphologic Relations Developed Between Plants Roots and Arbuscular Mycorrhizal Fungi (AFM) During The Establishment of Mycorrhizal Symbiosis: A Review. Open Acc J Agri Res: OAJAR-100010.
Received Date: 21 September, 2018; Accepted Date: 10 October, 2018; Published Date: 25 October, 2018
1. Abstract
This review shows the anatomic and morphologic relations developed during the signaling/establishment of biotrophic mutualism symbiosis between plants and Arbuscular Mycorrhizal Fungi (AMF). The main objective is to help in the comprehension of mechanisms involved in the formation of the mutualistic complex between the roots and the AMF.
2. Keywords: Appressory; Arbuscule; Mutualism; Strigolactones
3. Introduction
The anatomy and the morphology of plants roots have achieved prominence in several branches of knowledge, notably in the Biological and Agronomic Sciences, as they are one of the main resources of the vegetal body related to the supply and the sustentation of the plants [1]. The agricultural practices of soil management need special attention in the relations of the roots of different plants with the different management used, because the health of the plants is dynamically linked to these delicate relations [2].
The difficulty of obtaining bibliographical contents, which are scarce and pulverized in the agronomic and biological areas, makes it difficult to train specialized personnel to study the relations between agricultural practice and applied botanic [3]. On the other hand, the expression of the anatomical and morphological organization of the plants roots in the diverse areas of Agronomy and of the Biological Sciences are ample, being many the studies that deal with the structural resistance of the vegetables to the microorganisms [4]. Management practices linked to monocultures allow the reproduction of certain microorganisms that cause damage to crops, being common the use of agricultural pesticides in order to minimize this problem [2].
Thereby, studies have been made with the aim of evaluating the possibility of a reduction of chemical products in the control of harmful microorganisms, ranging from research on the structural strength of the plant, passing by the dynamic vegetation front to the management, to the use of microorganisms considered beneficial to the plants [3]. On this last point, the Arbuscular Mycorrhizal Fungi (AMF) are important, because they colonize the root system of most plants, forming a symbiosis of the type biotrophic mutualistic [5]. This symbiosis is widely distributed in the plant kingdom, occurring in 83% of the plants Magnoliopsida (dicotyledons), in 79% of the Liliopsidas (monocotyledons) and in all Gymnosperms, which do not change the external aspect of the root [6]. In addition, the occurrence of symbiosis is widespread in most habitats, both in natural ecosystems and in ecosystems changed by man [7].
The AMF belong to the order Glomales, two suborders (Gigasporineae and Glomineae). In the suborder, Gigasporineae is to the family Gigasporaceae (with two genera, Gigaspora and Scutellospora). Already, in the suborder, Glomineae are to the families Acaulosporaceae (with two genera, Entrophospora, and Acaulospora), Archaeosporaceae (one genus, Archaeospora), Glomaceae (with one genus, Glomus), and Paraglomaceae (a genus, Paraglomus) [8]. Although mycorrhizal associations were described in 1885 by Frank, but only in 1922, Thaxter did the first review of this group of fungi, at the time called Endogonáceos, and recognized 24 species. In the 70’s, new and relevant concepts were consolidated to the Endogonáceos, with the creation of new genera and the recognition of 44 species [9]. Recent studies [10,11] have established important taxonomic concepts for the group, and these works were extensively increased by Morton. Currently, the Glomales’ taxonomy is receiving significant updates and revisions, mainly on the relationships of these organisms with their phytobionts and their phylogenetic relationships [8,12-15]. The methods of studies and taxonomic aspects (descriptions of species and identification keys) are reasonably consolidated in several works [16,17].
The mutualism manifests itself in the bidirectionality of the nutrient exchange, in which the plant provides carbohydrate to the fungus, while it provides water and nutrients, especially for the case of the phosphor [18]. Although the symbiosis is beneficial for the phytobionts, the effectiveness varies depending on the combination of the plant species and fungus involved in the association [18]. In this way, the relationships involved in the formation of this symbiosis, since the signaling between the phytobionts, the early stages of the colonization process, as well as possible changes promoted by the AMF in the anatomical and morphological structure of the roots [19], should be considered aiming to a complete understanding of the relationships between the symbionts. There is few information about such relationships, as well as about the anatomical and histochemical modifications produced by mycorrhizal infections in plant tissues [20]. Some authors report that the AMF does not cause morphological changes in the roots [21], however studies show that the AMF induces changes in the architecture [19,22], morphology [3,19,22,23] and anatomy [19] of the roots of several plant species.
3.1. Factors Involved in the Symbiosis
The sequence of steps that lead to the formation of the symbiosis seems to be common to the majority of plant species and these AMF, regardless of the effectiveness of the symbiosis [24]. The development of the symbiosis requires significant changes in both symbionts, which initiate the process through a signaling between them [25,26].
The process of formation of the symbiosis can be divided into 4 stages of development:
These phases describe the time sequence of events that occur in a process of infection and colonization of plants by AMF. However, the establishment of the symbiosis in the entire root is highly asynchronous, with the phases described occurring at the same time, from the moment that the fungus began the colonization of the root [27,28]. The understanding of the morphological and physiological changes that occur in the symbionts suggests that the symbiosis is the result of a series of events previously identified [29].
3.1.1. Signaling of The Plant and The Response of the Fungi
The formation of the appressory, during the pre-contact, involves the reorganization and attraction between the symbionts, whereas the survival of the fungus, after the germination of the spore, depends on a rapid colonization of the host plant [24]. The results of studies of the 50’s indicated that the spores of most species of fungi remained on the ground and could germinate spontaneously independent of the emission signals of the plants [30]. In addition, the spores could retain carbon enough to repeat the process of germination, especially in the case of spores of Gigaspora gigantean, which could germinate up to 10 times [31]. However, recent studies show that, in the absence of a potential host, the hyphae would show a limited growth by the use of resources stored in the spore [32].
On the other hand, several studies [28,33,34] show that the spores of the AMF require the presence of exudates liberated by the plants roots to have their germination initiated or suppressed, which indicates the existence of receptors in the spores that are responsible for changes in chemical composition that cause the beginning of the development of tube germination, grow through the soil. When approaching the roots, the fungus suffers modifications in its morphology, enhancing the growth and branching of the hyphae, in response to the increase of the concentration of the exudates liberated by the host plants roots. According to the same authors, such responses increase the contact between the roots and the hyphae [35]. Some studies indicate that the main factor that acts as a stimulant to the branching of the hyphae (branch factors) is the 5-deoxi-strigol (classified as a strigolactone), factor produced by both Liliopsidas as Magnolipsidas [36,37]. This is because the concentration of strigolactones in the exudates of the host plants roots of AMF is higher than that of the plants that have a low possibility of being colonized [37]. The active site presented in the fungus responsible for the perception of the 5-deoxi-strigol has not yet been identified [38]. However, the detection of this factor was seen in the exudates of the carrot roots moments before the start of the increase of the speed of the branching of the hyphae of Gigaspora rosea and Glomus intraradices that were close to the same. According to these authors, the presence of this factor induces the transcription of mitochondrial genes, that increase oxygen consumption and elevate the breathing and the status of energy necessary for the branching of the hyphae. In this way, from the detection of the branch factor 5-deoxi-strigol, presented in the exudates liberated by the plants roots, there is a vigorous production and branching of hyphae of the fungus (Figure 1) for the quick contact with the plants roots for obtaining carbon sources [34].
On the other hand, the responses of plants to the colonization of the roots by the AMF are different from those observed with other microorganisms [39], being that the defenses of the plants are suppressed during the process of formation of the mycorrhizal symbiosis [28,29]. The chemical nature of at least one signaling molecule of the plant involved in the development is known, while a few is known about the signaling of the fungus, its perception, and results in the plant [40]. Studies indicate that sections of roots near the hyphae of AMF with intense branch had an expression of the gene MtENOD11 responsible for ß-glucuronidase (GUS), this expression is not presented in infections caused by pathogenic fungi [41]. When the contact and penetration are allowed, the expression of GUS is restricted in the area where occurs the infection and in the infected cells [42,43], indicating that occurs suppression of the activity of the expression of GUS in areas already colonized by roots [44]. Thus, it is possible regions that are not colonized in the plant root become ready for the contact with the fungus because of the expression of GUS, at the same time, in regions already colonized, occur the suppression of this expression [43]. When the contact is made at an accurate area of penetration and colonization is established, the expression of the gene responsible by the GUS is suppressed by the cells in direct contact with the penetration of the fungus [45]. In this way, the intensity and distribution of expression of GUS could be an indicative of a detection by the plant of a compound released by the fungus [24].
The identification and characterization of mutant plants, that are unable to develop the mycorrhizal symbiosis, can contribute to the identification of additional signaling molecules of the plant that are responsible for the communication between the symbionts [46]. As an example, the mutant plants of Medicago truncatula do not show the ability to form symbiosis with nitrogen fixing bacterium (NOD), and are also unable to form mycorrhizal symbiosis (MYC), suggesting that both of the symbiosis have a common genetic mechanism of regulation, defined by the gene MtDMI2 [45] (Figure 2). Although the AMF are not able to colonize the mutant plants roots of M. truncatula, even so, the hyphae would suffer ramifications, and, before the contact, the expression of MtENOD11 is still induced to compatible levels with wild plants, indicating that the presymbiotic properties are not affected [41,47]. However, the proximity contact suppresses the expression of GUS due to a lack of functionality of the MtDMI2. In a similar way to what occurs with the AMF, in the process of pre- infection of the nitrogen fixing bacterium (NOD) of the genus Rhizobia, the expression of GUS is found in large sections of the roots before contact, occurring, however, suppression when the proximity contact, which would prevent the formation of nodules [44].
Different studies have shown that the activity of fungal molecules induces the expression of a number of genes in the early stages of the development of the mycorrhizal symbiosis, in a similar way to the process of protein synthesis of “plant’s defense”, involved in the formation of nodulations in roots from the colonization of nitrogen fixing bacterium (NOD), which would be an indication that the genes involved in the formation of these nodulations could be the same involved in the synthesis of proteins with functions predicted in transcription in response to a signal from the fungus [40,42,48,49]. The similarity in the expression (or suppression) between the two types of symbiosis suggests that the responses are part of a system of preparation for colonization of the roots, signaled between the symbionts.
3.1.2. The Formation of Appressory and Penetration in the Roots
The beginning of the symbiosis is morphologically marked by the formation of the appressory, being the first physical contact between the fungus and the plant, marking the place of entry of the AMF in the host plant. From the penetration of the tissue, it begins the development of intercellular and intracellular through the cortex. For the process of adaptation, morphological integration and function compatibility occurs between the cells of the plant and the hyphae of AMF, it is necessary a highly evolved molecular recognition and coordination among them [50].
The appressory development can be considered a successful result of the recognition of events in presymbiotic between the symbionts [51]. Structurally, appressory differs from hyphae because it is a plan with elliptical tips, which serve to adhere to the cells on the surface of the roots through a system still little known [50]. The morphological changes in the hyphae are signaled by changes in the gene transcription of the fungus [52]. Plants that are unable to form a symbiosis with AMF would not induce these changes in the transcription gene of the fungus, which would prevent the formation of appressory [51]. After the formation of the appressory and their physical contact with the roots, some of the changes occur in the host cells, so the process of colonization begins [43]. The first event is the repositioning of the cell's nucleus of the adjacent to the appressory, associated with the formation of a column with a hollow intercellular, composed of microtubules and microfilaments (Figure 2), which serve to coordinate the movement of the hyphae between the cells and the cell's nucleus to a opposite position to that of the appressory [53]. Finally, the hyphae penetrates the cortex region of the root and follows the route formed by the column, through the intercellular space [53].
Since then the infection occurs, because the preparatory action to infection is activated throughout the tissue root, since the cells of roots have a complementary activity that facilitates the penetration of the fungus [43]. The preparation of the column for the passage of hyphae between the cells is accompanied by the synthesis of a membrane, from the moment that the hyphae becomes intracellular, forming an interface between the fungus and the plant cell [43] (Figure 3).
3.1.3. The Development of the Arbuscule
The arbuscule are known to be the main feature of the formation of the mycorrhizal symbiosis, representing an extreme form of intimacy and compatibility [42]. The formation of the arbuscule inside of host cells is associated with drastic changes in the morphology and physiology of both symbionts [24]. The formation of the “symbiotic membrane” and the interface between the symbionts has been recognized by several authors as a common feature of the different species of AMF in the process of colonization and intracellular growth [42,43,53,54] (Figure 4).
In this process, which occurs inside of the cortical cells, the hyphae of the fungus differentiates from arbucules, which are the structures involved in the extension of the cell plasma membrane, resulting in an interface area arbuscule-cortical cell, responsible to transfer water and nutrients, especially phosphor, of the fungus to the plant [50]. The cytoskeleton of the cells colonized sustains rearrangements in order to enable the development of the arbuscule-cell interface [54,55], and this reorganization can be flagged as prior to penetration of the cell [54]. With this the architecture of the host cells suffers changes, as the change of the nucleus from a peripheral position to a central position [53], the vacuoles become fragmented and a extensive periarbuscular membrane is synthesized along the cell plasma membrane [56].
The intense activity of both symbionts in the colonized cells leads to the collapse of the arbuscule after a few days, leaving, however, the cell cortex intact so that you can host another arbuscule [24]. Processes are not well-known that trigger the input of the fungus in the cells, but some authors have observed that the formation of a radial gradient of sugar between the vascular tissue and the external layers of cells may be involved in the induction of the formation of the arbuscule [57]. However, the recent identification of Strigolactones as a stimulant of the presymbiotic ramifications of fungus [58,59], combined with the discovery of part of the biosynthetic path of these substances in colonized roots [60,61] indicate that these molecules could be linked to the intracellular ramification of the hyphae [60]. In a similar way, processes that lead to the collapse of the arbucule inside of the cells are not known [58]. The beginning of the collapse of the arbucule may be caused by endogenous fungus signaling and/or coordinated by this signaling [24]. The development of the arbuscule would also be regulated by the gene activity of both symbionts [62]. The genes involved in the expression of GUS, during the presymbiotic, would also be involved in the signaling of changes in the plant cell before the formation of the arbuscule [25]. On the other hand, in response to the changes promoted in the cells, whose signal would be capture by the receivers of the hyphae, genes of AMF would promote changes in the hyphae structure with the goal of developing the interface between the fungus and the host [63].
3.2. Modifications in Anatomy and Morphology of Roots after the Establishment of Symbiosis
The morphology of the root system is genetically determined and can vary between plant species and individuals in function of environmental factors such as water availability, nutrients and temperature [64], and the plasticity of the root system may also be influenced by the AMF [19]. The morphology of the roots influences in the rapid development of the root system, which is critical to the success of the establishment of the majority plants [3]. This fact is fundamental to the understanding of the effects of AMF on root development [19]. The main effect of colonization of roots by AMF would be the reduction of the cortex thickness of the plants roots, at the same time that would increase in most of the morphological parameters of the xylem of the roots [19]. In this context, the AMF, after the establishment of the symbiosis, would increase the size and number of cells of the metaxylem [65], which is one of the categories of the primary xylem whose cells that are conductive distinguish lately and present a larger diameter [66], and the secondary xylem [65]. On the other hand, AMF do not seem to have an effect on protoxylem [19], since it presents cells that differentiate first acquiring lignified secondary walls early, which decreases the possibility of AMF performance on the growth of these cells [67]. Thus, the decrease in cortex area seems to be directly related to the increase in the number of metaxylem cells and the secondary xylem of the plants inoculated with AFM [19,68].
The regulation of the development of mycorrhizal symbiosis can be achieved through the modification of endogenous levels of phytohormones, by regulating auxin levels in the fungus and / or host [57]. Changes in the hormonal balance of plants, due to the establishment of the mycorrhizal association, have been described in several plant species, both Liliopsidas and Magnolipsidas, as a function of the ability of the AMF to biosynthesize these phytohormones [69]. Examples of this fact are the studies of endomycorrhizal fungi, extracted from roots of Ophrys lutea (Orchidaceae) and maintained in culture medium for three weeks in the dark, which released high concentrations of Indol Acetic Acid (IAA - indole-3 -acetic acid) and indole-3-ethanol, revealing the ability of these fungi to synthesize hormones [70]. The identification of these compounds in the mycelium extract suggests the transfer of these compounds from fungi to the host plant during the establishment phase of symbiosis [70].
Roots colonized by AMF show an increase in the production of auxins and cytokinins, which would be involved in the increase or in the continuity of the growth of the cells of the conducting tissue, especially the size and number of the cells of the metaxylem and secondary xylem [71]. The establishment of symbiosis would lead to the production of biochemical flags that would activate the genes involved in the production of these phytohormones, while the same flags would be responsible for the formation of root nodules of leguminous plants colonized by Rhizobium sp. [71]. Studies have shown that the tissue responses of the roots of cv. mutant of alfalfa MN 1008, which performs the transcription of these flags in the absence of the symbionts, were identical to those of plant roots colonized by both AMF as by Rhizobium meliloti. In view of this, one can intuit that the presence of AMF would favor the constant differentiation of the tracheal elements of xylem (Figure 5).
On the other hand, there seems to be possible variable effects on root morphology, according to the AMF species and plant species involved in symbiosis, influencing the size and growth of xylem cells [72]. Experiments with the species Glomus fasciculatum and Glomus etunicatum show that they induced changes in the roots of 'Elsanta' and 'Cambridge Favorite' strawberry cultivars, but did not cause any changes in the root morphology of the Rhapsody cultivar, showing the variable effect of these species of AMF on different cultivars of the same plant species [22]. Roots of plants that were colonized by AMF may or may not display increases in longevity, depending on the plant species and fungi involved in symbiosis [68,73,74]. However, the morphological attributes that can be affected by the AMF, such as the ramifications and the diameter of the conductive tissue, have a direct influence on the increase in the longevity of roots [74,75]. In addition to increasing longevity, root colonization by AMF provides a rapid renewal of the root system, increasing the rate of replacement of roots that collapse [76].
3.3. Acquisition of Nutrients and Benefits
AMF obtain carbohydrates from their hosts and provide the plant nutrients, especially phosphate. In the case of phosphate, depending on the plant-fungus combination, the acquisition may be wholly or partially carried out by the fungus [18]. The metabolic pathway of nutrient acquisition begins with assimilation through hyphal-soil interface [77]. In the hypha, the nutrient is translocated to structures of the fungus inside the roots [78], where it is transferred to the plant, via arbuscule [79]. The route of transfer of carbohydrates from the plant to the AMF follows the reverse direction [78,79]. The benefits provided by AMF on plant development are associated with many mechanisms of action of these fungi, which act directly or indirectly on plants [80]. One of the positive effects of AMF is due to the presence of the external mycelium, which plays an important role in the absorption of slow diffusion nutrients, such as phosphor and potassium [81-83], increasing the nutritional content of plants [84,85]. Associated with this, modifications promoted by AMF in xylem structure, as increase in number and diameter of metaxylem and secondary xylem cells, promote increased absorption of nutrients, such as nitrogen, phosphor and potassium, translocated to the upper of the plant, culminating with the acceleration of growth [82].
Increased absorption and transport volume of nutrients, such as nitrogen, which is a constituent of proteins [86], phosphor [87], which is essential for cell division and the metabolism of photosynthesis, and potassium, which acts on the electric equilibrium of the cells and in the opening and closing of the stomata [86], it is vital for vegetative growth. This contributes to higher responses of the inoculated plants in terms of vegetative development.
4. Final Considerations
Although the understanding of the mechanisms involved in signaling and in the establishment of mycorrhizal symbiosis needs further clarification, in recent years several researchers have shed light on this area. The elucidation of events associated with signaling and with the processes of formation of the structures of colonization of the AMF and of the responses of plants to these processes, as well as of the changes promoted in the morphology of the tissues of the plants root and the acquisition of nutrients, are considered to be fundamental for the good understanding of the benefits generated by the AMF to the colonized plants.
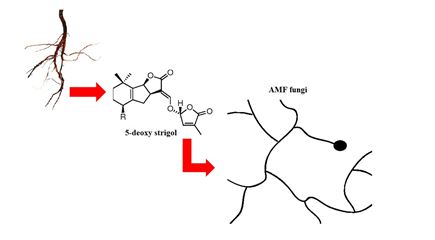
Figure 1: Releasing by the plant of strigolactones (5-deoxi-strigol) that induce the branching of hyphae of the AMF. Adapted from Paszkowski, 2006b [27].
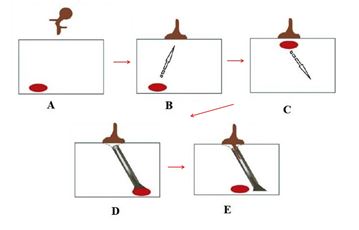
Figure 2: Preparation of host cells for penetration of the AMF: A - signaling; B - formation of the appressory; C - formation of the microtubule; D - formation of the microfilaments; E - penetration of the hyphae into the cells. Adapted from Paszkowski, 2006b [27].
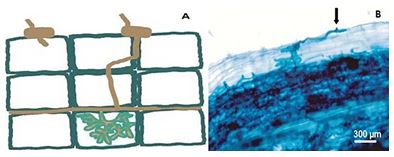
Figure 3: Formation of the appressory (A) and point of contact (arrow - B), the development and penetration of hyphae in the root cortex. Adapted from Paszkowski, 2006a [24].
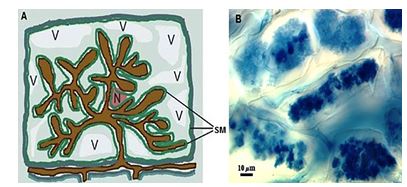
Figure 4: Schema that shows the formation of the arbuscule (A): N - nucleus; V - vacuole; SM - symbiotic membrane. (B) - arbuscule formed inside the cells of the roots of peach tree. Source: (A) - Adapted from Paszkowski, 2006b [27]; (B) - Photo: Itamar Garcia Ignácio (EMBRAPA Agrobiologia).
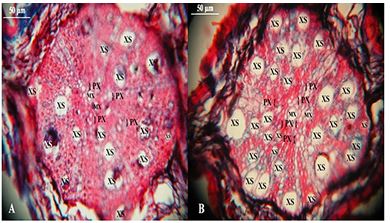
Figure 5: Cross-sections of second order roots peach plant without inoculation (A) and inoculated with AMF (B). MX - Metaxylem; PX - Protoxylem; XS - Secondary Xylem. Photos: José Luis da Silva Nunes.
Citation: Nunes JLS (2018) Anatomic and Morphologic Relations Developed Between Plants Roots and Arbuscular Mycorrhizal Fungi (AFM) During The Establishment of Mycorrhizal Symbiosis: A Review. Open Acc J Agri Res: OAJAR-100010.