Current Research in Psychiatry and Brain Disorders
Review Article
Nervous System and Cobalamin Deficiency
Khellaf S1*, Boulefkhad A2, Boudraa B2, Semra H2, Serradj F2, Boumala N2, Benhamada S2, Fekraoui BS2, Sifi Y2, Zahem AM2, Hamri A2 and Bouali F1
1Internal Medicine department, Khelil Amrane University Hospital Center, 06000, Bejaia, Algeria
2Neurology department, Dr Benbadis University Hospital Center, 25000, Constantine, Algeria
*Corresponding author: Khellaf S, Internal Medicine department, Khelil Amrane University Hospital Center, 06000, Bejaia, Algeria, Tel: +213795082200; E-mail: saddek.khellaf@gmail.com
Citation: Khellaf S, Boulefkhad A, Boudraa B, Semra H, Serradj F, et al. (2019) Nervous system and Cobalamin deficiency. Curr Res Psychiatry Brain Disord: CRPBD-100005
Received date: 05 December, 2019; Accepted date: 12 December, 2019; Published date: 19 December, 2019
Abstract
Cobalamin or Vitamin B12 deficiency is common and underdiagnosed in adults. It should be looked for, in addition to the classical hematological presentation, in patients with suggestive neurological signs such ataxia, paresthesia or cognitive impairment, particularly in populations at risk, such as elderly, alcoholic, vegetarian or malnourished. The main causes of this deficiency are the food cobalamin malabsorption syndrome, Biermer's disease and, less frequently, intestinal malabsorption and lack of intake. The understanding of the metabolism of this vitamin, not to mention the mechanism of neurological damage, is essential but still incomplete. However, current knowledge makes it possible to develop diagnostic and therapeutic approaches. The diagnosis is retained either by a biochemical test expressing the decrease in vitamin B12 stores, or retrospectively after improvement under substitution treatment based on vitamin B12 supplementation.
Keywords: Anemia; Cobalamin; Nervous System Diseases; Subacute Combined Degeneration; Vitamin B12
Introduction
Cobalamin or vitamin B12 deficiency is defined according to several different definitions in the absence of standardized and reproducible criteria, see (Table 1) [1]. This deficiency is quite common among adults and often underdiagnosed because of its insidious clinical presentations; its prevalence would be about 15 to 20% in the general population [2], ranging between 5 and 60% depending on the definition used, it is higher among the elderly (30 to 40%) [3]. Although the most suggestive clinical picture is Subacute Combined Degeneration (SCD), many cases of deficiency have little or no symptoms. requiring the practitioner to have a thorough knowledge of various vitamin B12 deficiency symptoms to best prevent the potentially serious consequences.
Metabolism
Food intakes and reserves: Made exclusively from animal products (offal, especially beef, fish, eggs and dairy products), the daily intake of vitamin B12 varies between 5 and 7 mg for a recommended dose of 2.5 to 3 mg / d [4, 5]. Mainly stored in the liver, its reserves are estimated between 2 and 5 mg, which corresponds to about 1000 days of intake; this reserve contributes to the diagnostic difficulty of the serum cobalamin assay and explains the delay between the intake deficit and the beginning of the cell deficit [6].
Digestion and absorption: Intestinal absorption of vitamin B12 is performed through two separate systems, which highlight the crucial roles of inadequacy, particularly the role of gastric and exogenous pancreatic functions, as well as the integrity of the ileal mucosa [6].
Intrinsic Factor (IF) Dependent System: Ingested cobalamin detaches itself from dietary proteins in the stomach under the action of gastric juice and pepsin, then it binds to a protein carrier called haptocorrine, secreted by the salivary glands and gastric cells. Then, in contact with the biliary and pancreatic secretions, this complex is lysed in the duodenum. It is at this level that bile secretion of the enterohepatic cycle occurs. Finally, IF secreted by gastric cells binds to vitamin B12, unlike haptocorrine, IF protects cobalamin from ileal bacterial catabolism and binds specifically to the terminal ileal cell by a receptor called cubuline. It follows a calcium-dependent mechanism of internalization by endocytosis. It is clear that this specific and efficient absorption system is however saturable [6].
IF independent system: Allows the diffusional absorption of 1 to 5% of the ingested dose of vitamin B12, it is insufficient to provide the body with the daily required dose during a balanced diet, but it is non-saturable, allowing recourse to oral substitution [6].
Transport: Transcobalamin I, II and III participate in the serum transport of vitamin B12. Only Holotranscobalamin II (Transcobalamin) seems to have physiologically an important role, thus allowing vitamin intake to the cell metabolism pathways. That is why the serum dosage of Transcobalamin is more accurate than the overall dosage of vitamin B12 [7].
In the cytoplasm: Vitamin B12 mainly acts as a coenzyme in the form of methyl-cobalamin catalyzing the action of methionine synthetase allowing the conversion of homocysteine (HC) to methionine, but also the conversion of methyl-tetrahydrofolate to tetrahydrofolate (THF) which can be used in the synthesis of purine and pyrimidine bases. As a result, methyl-cobalamin deficiency will cause methionine deficiency with an increase in HC, thus blocking DNA replication by decreasing THF, leading to a nucleo-cytoplasmic maturation asynchronism expressing megaloblastosis on myelogram [8, 9].
In the mitochondria: In the form of intra-mitochondrial adenosyl-B12 which allows the conversion of propionyl-CoA to methylmalonyl-CoA and finally succinyl-CoA, an intermediate of the Krebs cycle. Whose consequences of deficiency are, in addition to succinyl CoA deficiency, the accumulation of methyl malonic acid (MMA) [8, 9].
MMA and HC, thus constitute two metabolic markers which will increase in case of cellular deficiency in vitamin B12.
Elimination: Elimination of serum cobalamin excess is renal [10]. Serum levels are modulated by liver reserve utilization, renal tubular reabsorption and enterohepatic circulation [10, 11].
Pathophysiology: The neurological signs related to vitamin B12 deficiency are linked to complex and still imperfectly understood mechanisms. they would be due to a disorder of the methylation of the myelin sheaths with abnormalities of the nerve conduction, either by decrease of methionine, and thus of its metabolite S-adenosyl methionine (SAM) which participates in basic myelin protein composition, or by accumulation of methylmalonic acid, which is a toxic fatty acid for myelin [12-14].
More recent studies offer a very different explanation; the clinical and histological manifestations of B12 deficiency may be the result of a regulatory phenomenon that will amplify the neurotoxic effect of many cytokines but also negatively affect the restorative action of certain neurotrophic factors [15, 16]. The resultant hyper homocysteinemia would be an independent cerebrovascular risk factor, associated with atherosclerosis and cerebrovascular accidents. However, the link is not yet well documented and remains controversial [1, 17]
In general anesthesia, patients with sufficient body reserves of vitamin B12 may maintain cellular functions after exposure to nitrous oxide (N2). On the other hand, patients with limited or low vitamin B12 reserves, oxidation of the core of vitamin B12 by nitrous oxide (N2O) may be sufficient to render methyl cobalamin inactive, to inhibit the conversion of HC to methionine and exhaust the contribution of SAM [18, 19].
Finally, neurological disorders sometimes appear after insufficient replacement therapy or following folate treatment. This is the theory of "folate trap" where the contribution of folate mobilize the last stocks of vitamin B12 in favor of the hematological line rather than neurological, thus maintaining the synthesis of nucleic acids to the detriment of the formation of methionine and therefore myelin [20].
Etiologies
Etiologies of vitamin B12 deficiency are intimately related to the stages of its digestion and its metabolism (Figure 1). The main causes of vitamin B12 deficiency in adults are represented by the Food-cobalamin malabsorption syndrome (60%), Biermer disease (18%), intestinal malabsorption (6%) and lack of intake (2%) [1, 21].
Lack of intake: Outside a strict exclusion regime like vegan, or in an elderly or already malnourished person, vitamin B12 deficiency is extremely rare in healthy adults [1].
Absorption abnormalities: The most common etiology of cobalamin malabsorption in adults is deficits in exocrine pancreatic function following chronic pancreatitis (usually alcoholic) or after pancreatectomy [22]. Other causes include gastrectomies and surgical resection of the terminal small bowel (<5%), and even more rarely (<2%): Crohn's disease, lymphoma, tuberculosis, amyloidosis, scleroderma, celiac disease; taking colchicine (by inhibiting the expression of cubulin at the apical pole of the enterocyte cell) [23] or cholestyramine [1].
Biermer disease: It is an autoimmune disease affecting the gastric mucosa, especially fundic (classic autoimmune A-type atrophic gastritis), and by the presence of various antibodies (AB), especially in the blood plasma and gastric secretions. AB anti FI (sensitivity: 50%, specificity:> 98%) and AB anti gastric parietal cells (sensitivity:> 90%, specificity: 50%) [24]. This disease is further characterized by the presence of a malabsorption of B12 corrected by the addition of FI during the Schilling test (specificity> 99%) which is no longer available [1]. Clinically, one of the particularities of Biermer's disease is to be associated with many autoimmune disorders: vitiligo, dysthyroidism, Addison's disease, Sjögren's syndrome, etc. Exceptional associations with chronic hepatitis C (treated with interferon alfa) have also been reported [1, 24]. Once the diagnosis is made, gastric fibroscopy with fundic biopsies remains systematic and serves as a reference examination for subsequent systematic follow-up (possible complication with neoplasia).
Food-cobalamin malabsorption: This syndrome is characterized by an inability to release B12 from food proteins and / or intestinal transport proteins, especially in case of hypochlorhydria while the absorption of B12 "unbound" is normal. In practice, the non-availability of Schilling tests (standard and modified) makes the Food-cobalamin malabsorption syndrome a diagnosis of elimination which rests on two aspects:
Three criteria must be present: a serum vitamin B12 concentration of less than 200 μg/ML, the absence of intrinsic factor antibodies (or normal standard Schilling test with abnormal "modified" Schilling test) and finally the absence of nutritional vitamin B12 deficiency (intake > 2 μg per day) [1, 21].
The existence of a predisposing factor for this deficiency: such as atrophic gastritis, chronic infection with Helicobacter pylori, gastrectomy, gastric bypass, vagotomy; Exocrine pancreatic insufficiency (chronic ethylism, cystic fibrosis); Taking anti-acids (antihistamines 2 or proton pump inhibitors) or biguanides (metformin); microbial overgrowth Sjögren's syndrome, Scleroderma, age-related "Idiopathic" deficiency or homozygous haptocorin congenital deficiency [1, 25, 21].
A and B: T2 Medullary MRI showing the V-inverted sign as a hypersignal of the posterior cords on axial (A) and an extended medullary cervical hypersignal on sagittal (B) [60].
C and D: T2 FLAIR Brain MRI axial sections. Showing symmetrical hypersignals of the dorsolateral brainstem regions (C) [49]. And diffuse and symmetrical hypersignal of the periventricular white matter in a 45-year-old woman with progressive cognitive decline (D) [9].
Iatrogenic causes: Nitrogen protoxide (NO) used in anesthesia is a strong oxidizing agent that irreversibly oxidizes the cobalt atom of vitamin B12, rendering methyl-cobalamin inactive [19]. Similarly, vitamin C supplementation is reported to induce cobalamin deficiency by a similar mechanism [26, 27]. Finally, when the patient's prescription mentions a folate substitution.
Without a vitamin B12 substitution, the mechanism of the folic trap must be strongly evoked in the presence of any compatible clinical sign, despite the absence of anemia or macrocytosis [28, 29].
Hereditary diseases of vitamin B12 metabolism: These deficits are neonatal revelation and usually do not affect adults, such as deficiency in IF (in the form of juvenile and familial forms of Biermer disease), in cubuline (as in Imerslund-Gräsbeck disease) or in transcobalamin II, and exceptionally deficits in intracellular enzymes involved in the biosynthesis of the active forms of cobalamins: adenosyl- and methyl-cobalamin [1].
Clinical presentations
Cobalamin deficiency is very common in specific groups of the population. In fact, the risk of vitamin B12 deficiency is high among vegetarians, malnourished, infants, pregnant and lactating mothers, as well as among the elderly and institutionalized persons [30, 3]. This deficiency can take several years to reveal itself. Twenty-six to sixty-six percent of patients develop neurological and extra neurological manifestations that may be present even in the absence of anemia. The main manifestations are the Subacute Combined Degeneration (SCD), peripheral neuropathies, dysautonomic disorders, dementia and other rarer disorders [31, 32]. These different syndromes can be associated in the same patient, including SCD and peripheral neuropathies in about 50% of cases [33].
Subacute Combined Degeneration: Represents the most classic presentation, though rarely seen today [21]. It associates posterior cordial syndrome (PCS) and pyramidal syndrome. The first signs are those of the PCS, with paresthesia (tingling, numbness and pain) predominating in the lower limbs and sometimes in the trunk and upper limbs, a sign of Lhermitte, a proprioceptive ataxia with sometimes isolated apallesthesia. This PCS in extension is followed by the occurrence of a pyramidal syndrome manifested by tetra or paraparesis, spastic hypertonia especially in the lower limbs, vivid tendinous reflexes and a Babinski sign. It is sometimes difficult to clinically distinguish these 2 syndromes, the contribution of evoked potentials and medullary magnetic resonance imaging (MRI) is of great help in this case [34].
peripheral neuropathies: They account for 30 to 50% of the neurological complications of B12 deficiency [31, 35, 34, 32] and are present in 6-8% of cases in cohort studies for all causes of neuropathies combined [33, 36]. These are mainly sensory neuropathies, of moderate intensity, acute or subacute, non-ataxiant, symmetrical and length dependent. Sometimes they are sensory-motor polyneuritis, electrically axonal polyneuropathy, rarely demyelinating or mixed, usually dominated by paresthesia and deep sensibility disorders. According to Franques et al. [Table 2], Several contextual elements with acute or subacute sensory neuropathy and independently of biological markers, should evoke a cobalamin deficiency in order to start substitution therapy as soon as possible. case of effectiveness will be the strongest argument in favor of this hypothesis [37].
Dysautonomic disorders: Dysautonomia is not uncommon during B12 deficiency as it is estimated at 22% of cases and it is inaugural once in two [38]. It is mainly orthostatic hypotension or genitourinary sphincter disorders [39].
Other neurological signs: Cobalamin deficiency could be the most commonly organic disease associated with dementia. Nearly 40% of patients with dementia, Alzheimer's disease in particular, have low serum vitamin B12 levels [40]. In France the high health authority recommends the dosage of vitamin B12 in the balance sheet of Alzheimer's disease [41]. Other authors propose to extend these recommendations to the assessment of all dementias. However, in the work of Andres et al., 30% of neuropsychiatric manifestations do not respond to well-conducted cobalamin therapy.
[25, 1]. Painful spinothalamic syndrome, cerebellar ataxia or retrobulbar optic neuritis are also possible. Other manifestations, such as Parkinson syndrome, depression, manic states, psychoses, obsessive-compulsive disorder and sleep disorders, have been described, but the causal relationship has not yet been demonstrated [21].
Extra neurological manifestations
Complementary exams
Biology
Blood Formula Count: Vitamin B12 deficiency is commonly responsible for megaloblastic anemia, characterized in its historical and booklet form by frank macrocytic anemia (mean corpuscular volume, MCV, greater than 110 μm3), normochromic, arterenative with megaloblastosis medullary (giving a "Blue marrow"). Leukopenia and moderate thrombocytopenia are associated [1, 42, 43]. However, the haematological picture of vitamin B12 deficiency is most often incomplete, sometimes even severe (deep anemia <6 g / dl, pancytopenia) that may be life-threatening, or even atypical (haemolytic anemia, pseudo thrombotic microangiopathies) [12].
Dosage of Vitamin B12: As already mentioned, there are currently no formal biological criteria for the diagnosis of vitamin B12 deficiency. Nevertheless, most recent studies apply the criteria listed in [Table 1] to diagnose vitamin B12 deficiency [1]. Dosage of cyanocobalamin (cobalamin bound to transport proteins) reflects the total level of vitamin B12 circulating in the blood. Only the fraction bound to transcobalamin II, called transcobalamin, about 6 to 20%, is bioavailable and therefore biologically active. The disadvantages of this method are the variations of the reference intervals depending on the different immunoassays used. False positives are possible if haptocorin is decreased (pregnancy) and false negatives are possible when it is increased (myeloproliferative neoplasia, hepatoma).
Dosage of methyl malonic acid:More sensitive than vitamin B12 with sensitivity close to 100%, but its specificity is also subject to debate. While it also increases in case of kidney failure, MMA is currently considered in research as the reference value. Its high cost and limited availability make it not recommended in care practice as first intension [44].
Determination of homocysteine (HC): Increased in case of vitamin B12 deficiency, this marker is considered more sensitive than the dosage of vitamin B12, however with a bad specificity. False positives are possible during deficiencies in folic acid or vitamin B6. In addition, HC rate is increased in cases of renal failure, active smoking, alcohol consumption and coffee consumption [45].
Dosage of Transcobalamin: It is the Holotranscobalamin II-B12 complex, and represents the bioavailable part of vitamin B12. Its values vary little during a day (measurement can be done fasting or not) but faster than other biomarkers after a change in vitamin B12 intake (from 2 days). It increases in case of renal insufficiency, though to a lesser extent than the MMA and HC and it does not vary during pregnancy [7].
Normal rate of vitamin B12: Opposing to popular belief, a normal serum vitamin B12 test does not eliminate a cellular deficiency. In fact, 54% of responders clinically and biologically responding to cobalamin substitution, had a pre-therapeutic dosage of normal vitamin B12 [46]. In addition, vitamin B12 may even be increased or falsely normal in case of deficiency in certain situations such as chronic renal failure, liver diseases, myeloproliferative syndromes or intestinal colonization by certain bacteria producing vitamin B12 "like" substance [47,48]. Cell metabolites of B12, homocysteinemia and serum methyl malonic acid can also be taken as the default, as they are normal in 50 and 25% of cases respectively. Thus, if we limit ourselves to biological markers, 63% of responder patients would not be treated [46].
Neuroimaging : MRI may show a posterior cordial hyperintensity in T2 (Figure 3); classically known by the inverted V sign in axial section, most often cervicodorsal, associated or not with a medullary edematous swelling in T1, sometimes interesting the brainstem [49]. Images of diffuse leuko encephalopathy generally symmetrical, however totally nonspecific are also described [9]. The predominance of abnormalities in the white matter and their nonspecific nature often raise the difficulty of differential diagnoses, including degenerative and /or demyelinating diseases with bilateral posterior hyperintensity: infectious myelitis, myocardial infarction, multiple sclerosis (MS), acute disseminated encephalomyelitis (ADEM), acute transverse myelitis, and copper deficiency myelopathy [52]. However, several observations of obvious clinical abnormalities with no evidence of detectable MRI have been reported, which may indicate late radiologic abnormalities [50, 51].
Neurophysiological explorations
Prolonged somatosensory evoked potentials, particularly by lower limb stimulations indicative of posterior medullary cord injury, are much more frequently observed than abnormalities of peripheral sensory conduction strictly speaking [53, 54]. However, electroneuromyographic (ENMG) results, according to several studies, favor an axonal sensory involvement in 22 to 70% of cases, demyelinating in 2 to 17% of cases, mixed in 18 to 67% of cases [34, 35, 55].
The Diagnostic approach
As shown in (Figure 3), this approach is intended primarily realistic, especially in the elderly, avoiding invasive or systematic or even "unnecessary" explorations, such as systematic myelogram or gastroscopy of principle [1]. Indeed, in front of a neurological and or extra neurological presentation evoking a cobalamin deficiency, especially in a population at risk (see above). It is desirable to claim biologically this vitamin B12 deficiency, by performing a determination of vitamin B12 and homocysteine ??levels.
Once the clinical or paraclinical picture of vitamin B12 deficiency is strongly evoked (Table 1), it will be necessary to start by looking for clinical and biological stigmas of undernutrition or malabsorption (weight, albuminemia, dietary survey, diarrhea or steatorrhea) as well as certain iatrogenic causes (see above) and start the appropriate etiological and replacement treatments.
If this first assessment is negative, then Biermer's disease should be mentioned, especially in the presence of a field of autoimmunity. It will be appropriate in the first place, to look for the presence of anti-IF antibodies and gastric parietal cells serum, elevation of gastrinemia (or chromogranin A). Then and only at this stage, perform a gastroscopy with systematic biopsies. Lifetime substitute therapy will be offered if Biermer's Disease is confirmed.
Finally, if none of these etiologies is found, the diagnosis of food-cobalamin malabsorption syndrome will be retained and treated so, while looking for circumstances or contributing factors (Table 2) or even retain its "idiopathic" character in case of good response after one month of oral treatment test.
(Figure 3)
Therapeutic management
Currently, there is no precise recommendation regarding the treatment of Cobalamin deficiency neurological disorders. Although 2000 mg orally is as effective as 1000mg by intramuscular, even in Biermer disease [56], the effectiveness of oral cobalamin therapy on neurological manifestations has not been sufficiently documented to date, It is therefore always recommended to use the parenteral route in this category of patients [21]. whatever the route of administration, vitamin B12 substitution is not directly toxic, but there are rare cases of anaphylaxis [57]. Not to mention that the detection of vitamin B9 and iron deficiency must be done at the time of diagnosis because they are often associated. The treatment consists of 2 phases :
Charge treatment: Parenteral (subcutaneous or intramuscular), to bring vitamin B12 to already deficient cells and to initiate a reserve. At the dose of 1000mg / day for 7 days than 1000mg / week for 1 month. If there is a diagnostic doubt about a vitamin B12 deficiency, clinical evaluation can be used after a load treatment as an additional aid. Sometimes it is enough to treat the cause without charge treatment to correct the deficit.
Maintenance treatment: The aim is to provide the body with the equivalent of its vitamin B12 needs. At the dose of 1000 mg/month until correction of the cause or for life in Biermer's disease. In principle, no biological monitoring is necessary if the compliance is good. However, it is important to draw attention to the potential risk of long-term nonobservance and therefore to the need to discuss the benefits and risks of the oral route based on the patient's profile and ability to adhere to treatment [25].
Evolution
The majority of patients respond within 3 months of substitution [37], sometimes the state of some patients improves up to 12 months or even 3 years. The risk of sequelae depends mainly on the delay in the introduction of vitamin therapy [32]. Thus, 50% of residual lesions due to axonal loss are reported in late diagnosis forms [14]. Although complete healing of dementia seems possible [58], data concerning the evolution of cognitive disorders under treatment remain controversial [28]. Radiologically, the complete reversibility of the images is correlated with a cure without sequelae. However, at the too late stage of axonal degeneration and gliosis, the lesions are irreversible and then abnormalities in imaging persist.
Conclusion
Neurological disorders due to cobalamin deficiency are polymorphic and may occur outside any hematological context. For this reason and independently of the etiology, the dosage of vitamin B12 is suggested, and if necessary, that of its early markers such as homocysteine or even methyl-malonic acid, without justifying a diagnostic delay, and therefore a delay of vitamin substitution, which represents the main prognostic factor of this "benign" disease.
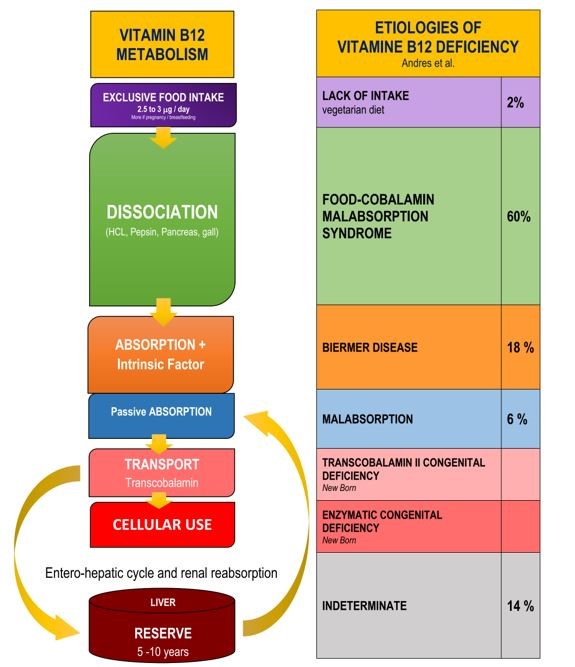
Figure 1: Etiologies of vitamin B12 deficiency corresponding to the different stages of its metabolism according [1,16].
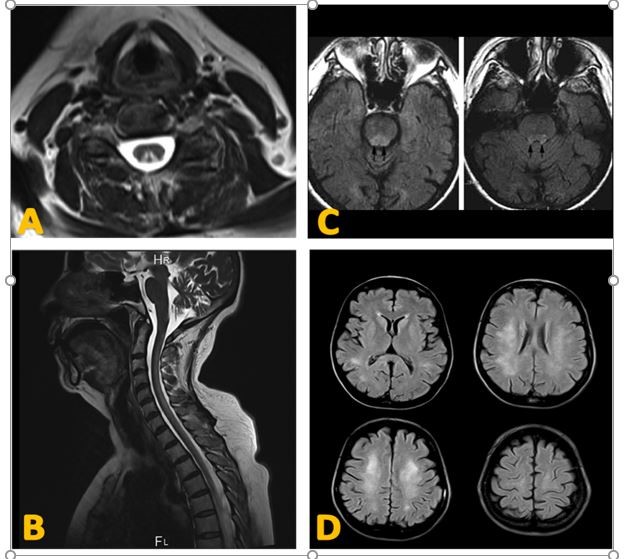
Figure 2: Neuroimaging aspects of Cobalamin deficiency [9, 49, 60].
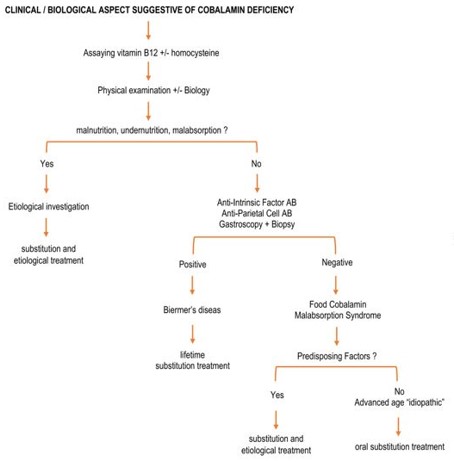
Figure 3: Diagnostic approach to vitamin B12 deficiency [1, 25].
|
Vitamin B12 < 200 pg/mL (or 150 pmol /L), twice |
|
Vitamin B12 < 160 pg / ml |
|
Vitamin B12 < 200μg/ml |
|
and Total Homocysteine < 13μmol /L or Methyl Malonic > 0.4μmol / L |
|
(without renal insufficiency or folate and vitamin B6 deficiency nor a mutant of methyltetrahydrofolate reductase). |
Table 1: Different proposals for vitamin B12 deficiency definition [1].
|
Context |
Elderly |
|
Undernutrition, vegetarianism |
|
|
Isolated prescription of folic acid without vitamin B12 |
|
|
General anesthesia by nitrous oxide |
|
|
Pancreatic insufficiency |
|
|
Crohn's disease |
|
|
Prescription of proton pump inhibitor or Biguanides |
|
|
Syndrome |
Axonal neuropathy, sensitive, acute or subacute |
|
Subacute Combined Degeneration |
|
|
Biological disorder |
Normo-, macro- or microcytic anemia (iron deficiency) |
|
Significant elevation of homocysteinemia (not very specific) |
|
|
Elevation of serum or urinary methyl malonic acid |
|
|
Biermer's autoantibodies or hypergastrinemia |
|
|
Therapeutic response |
Clinical improvement within 3 months after the introduction of 2000 mg / day of oral vitamin B12. |
Table 2: Elements for vitamin B12 deficiency neuropathy despite normal serum assay; according to [37].
|
Manifestations |
Certain link |
Probable link |
|
Hematological |
Megaloblastic anemia |
- |
|
thrombocytopenia |
- |
|
|
leukopenia |
- |
|
|
pancytopenia |
- |
|
|
Intramedullary haemolysis |
- |
|
|
Thrombotic Pseudomicroangiopathy (rare) |
- |
|
|
Epithelial |
Hunter's glossite |
Digestive disorders |
|
- |
Vaginal atrophy |
|
|
- |
Urinary tract infections |
|
|
- |
Cutaneous ulcers |
|
|
Vascular |
Deep vein thrombosis |
Atherosclerosis |
|
|
|
|
|
|
|
|
|
Others |
- |
low fertility |
|
- |
Abortions |
Table 3: shows the main events reported in the literature [21].
Citation: Khellaf S, Boulefkhad A, Boudraa B, Semra H, Serradj F, et al. (2019) Nervous system and Cobalamin deficiency. Curr Res Psychiatry Brain Disord: CRPBD-100005