Annals of Medical & Surgical Case Reports
Review Article
Anti-Infectious Activities for Bacterial Zn2+-Induced Peptidoglycan Autolysinsand Viral Zinc-Finger Fusion Proteins against Bacterial and Viral Infections
Ishida's T*
2-3-6, Saido, Midori-Ku, Saitama-Shi, Saitama-Ken, Japan
Volume 2020; Issue 011
*Corresponding author: Tsuneo Ishida, 2-3-6, Saido, Midori-Ku, Saitama-Shi, Saitama-Ken, Japan.
Citation: Ishida’s T (2019) Anti-Infectious Activities for Bacterial Zn2+-Induced Peptidoglycan Autolysins and Viral Zinc-Finger Fusion Proteins against Bacterial and Viral Infections. Ann Med & Surg Case Rep: AMSCR-1000028
Received date: 05 November, 2019; Accepted date:18 November, 2019; Published date: 03 January, 2020
Abstract
Anti-infectious activities of bacterial Zn2+-induced peptidoglycan autolysins and viral zinc-finger fusion proteins are respectively discussed against both bacterial and viral infections. Bacterial peptidoglycan (PGN) autolysin AmiA for S. aureus amidase is acted on PGN binding and cleavage that amiA distinguishes PGN mostly by the peptide, and cleavage is facilitated by a zinc-activated molecule. The autolytic activity of the recombinant amidase of the Aas (autolysin/adhesin of Staphylococcus saprophyticus) is inhibited and is necessary for the C-terminal GW repeats, not the N-terminal repeats. AmiB catalyzes the degradation of PGN in bacteria, resulting in a marked increases of sensitivity to oxidative stress and organic acids. Amidase activity of amiC controls cell separation and PGN fragments release. In these autolysins, zinc-dependent PGN autolysin of amidases may be enhanced and induced anti-bacterial activities.
Lytic amidase autolysin LytA associates with the cell wall via its zinc-binding motif.The LytB PGN hydrolase responsible for physical separation of daughter cells cleaves the GlcNAc-β-(1,4)-MurNAcglycosidic bond of PGN building units. LytC, LytD, and LytF are expressed in the same subpopulation of cells and complete flagellar synthesis. Thus, autolysin mediated bacteriolys-is induced bacterial cell death can contribute to the bactericidal activities.
Enveloped viruses enter cells and initiate disease-causing cycles of replication that in all cases virus-cell fusion is executed by one or more viral surface glycoproteins denoted as the fusion protein, in which the structure and mechanisms on viral membrane fusion protein are important problems. The novel EBV-induced zinc finger gene, ZNFEB, including its intron less locus and human protein variants, controls entry and exit from cell cycling in activated lymphocytes.The designed polydactyl zinc finger protein is prepared consiting HIV-1 type integrase fused to the synthetic zinc finger protein E2C that the integrase-E2C fusion proteins offer an efficient approach and a versatile framework for directing the integration of retroviral DNA into a predetermined DNA site. The zinc-finger antiviral protein (ZAP) specifically inhibits the replication of certain viruses and promotes viral RNA degradation. Zinc finger protein Tsip1 controls Cucumber mosaic virus (CMV) RNA replication. The zinc-finger protein ZCCHC3 binds RNA and facilitates viral RNA that ZCCHC3 is a co-receptor for the retinoic acid-inducible gene-1 (RIG-1) and antigen MDA5 which is critical for RIG-1 like receptor (RLR)-mediated innate immune response to RNA virus.The membrane fusion reaction, membrane interaction, conformational changes of specialized virus envelope proteins, and refolding reactions of specific fusion proteins have been discussed. Anti-viral activities of zinc-finger protein, zinc-binding domain, and membrane fusion protein are recognized by which highly diverse fusion proteins have converged on the same overall strategy to mediate a common pathway of membrane fusion, causing to lead enhancement of the anti-viral activity.
Keywords: Bacterial PGN autolysin; PGN autolysin amidase; Viral zinc-finger fusion protein; Viral entry replication
Abbreviations
Aas : autolysin/adhesin of Staphylococcus saprophyticus
ABC : ATP-binding cassette
APC : antigen presenting cell
A. stephensi : Anopheles stephensi
B. abortus : Brucellaabortus
B. subtilis : Bacillus subtilis
CBDs : cell wall binding domains
CBPs : choline binding proteins
C. difficile : Clostridium difficile
CKD : Chronic Kidney Disease
CMV : Cucumber mosaic virus
E. coli : Escherichia coli
E. faecalis : Enterococcus faecalis
ETEC : Entero-toxigenic E. coli
Eps : Zinc dependent endopeptidases
FnBPs : fibronectin-binding proteins
Gas : group A streptococcus
GelE : gelatinase
HCV : hepatitis C virus
HD : hemodialysis
HIV-1 : Human immunodeficiency virus type 1
M. catarrhalis : Mora-xellacatarrhalis
MCPs : Metallocarboxypeptidases
MIBRs : most probable immuno-protective B-cell epitope regions
MRB : multidrug bacteria
ORSs : oral rehydration solutions
ORT : oral rehydration therapy
P. aeruginosa : Pseudomonas aeruginosa
PBP2a : penicilline-binding protein2a
PGN : peptidoglycan
PGRPs : peptidoglycan recognition proteins
PSP : plasmid stabilization protein
RIG-1 : retinoic acid-inducible gene-1
RLR : RIG-1 like receptor
ROS : reactive oxygen species
Sags : super-antigens
SasG : S. aureus surface protein
S. aureus : Staphylococcus aureus
SBP : solute-binding protein
SEB : staphylococcal entoxin serotype B
SOD : superoxide dismutase
S. pneumonia : Streptococcus pneumoniae
SSP : stable signal peptide
TBVs : transmission-blocking vaccines
Tsip1 : Tsi1?interacting protein 1
VRE : vancomycin-resistant Enterococcus faecium
ZAP : zinc-finger antviral protein
ZBD : zinc-binding domain
ZBL : zinc binding lipoprotein
ZNFEB : EBV-induced zinc finger gene
ZnuA : Zinc uptake A
Introduction
Zinc is the second most abundant trace metal with human body 2?3g, 90% in muscle and bone, and 10% other organs include prostate, liver, the gastrointestinal tract, kidney, skin, lung brain, heart, and pancreas in humans that cellular zinc underlies an efficient homeostatic control that avoids accumulation of zinc in excess. Zinc influences apoptosis by acting on several molecular regulators of programmed cell death and zinc deficiency caused by malnutrition and foods with low bioavailability, aging, certain diseases, and deregulated homeostasis is a far more common risk to human health without intoxication [1]. The role of zinc in cell death has apoptosis that the influence of zinc on apoptosis is tissue/cell type, zinc concentration, and expression of zinc transporters and zinc-binding proteins. Host zinc homeostasis changes in response to bacterial infections, including production of metal sequestering proteins and bombardment of bacteria with toxic level of zinc at host-pathogen interface [2]. Apoptosis is defined as cell death activated by an internally controlled suicide program that bacteria are able to trigger apoptosis, including the secretion of compounds such as protein synthesis inhibitions, pore forming proteins, molecules responsible for the activation of the endogenous death in the infected cell, and super antigens [3]. Regulation of apoptosis is essential for normal embryonic development and for homeostasis in adult tissue.
Zinc has a rather low toxicity and influences apoptosis by acting on several molecular regulators of programmed cell death which can inhibit apoptosis thereby either prolonging the survival of infected cells such that the production of progeny virus is maximized or facilitating the establishment of virus persistence. The influence of zinc on apoptosis is very complex that variables in this complex network are tissue and cell type, zinc concentration, expression of zinc transporters and zinc-binding proteins, oxidative or nitrosative stress, and the improvement of molecular opposing functions. The other, zinc deficiency in Chronic Kidney Disease (CKD) patients may be due to fecal excretion or decrease in its absorption that zinc concentrations were lower in hemodialysis (HD) patients compared to controls and Zn concentration 69.16 μg/dL of blood in HD patients, however, revealed no correlation among serum Zn concentration and anemia, serum parathyroid hormone concentration or pruritus severity in HD patients [4].
Zinc ion killing occurs chiefly by bacteriolyses of bacterial cell walls due to activated peptidoglycan (PGN) autolysins such as amidases, endopeptidases, and carboxypeptidase against bacteria [5]. PGN autolysins induced anti-bacterial vaccine activity may be enhanced by activation of zinc dependent PGN autolysins. PGN autolysins are bacterial peptidoglycan degrading enzymes that these muropeptides can be produced or modified by the activity of bacterial glycolytic and peptidolytic enzymes referred to as PGN hydrolases and autolysins which specific bacterial pathogens use PGN degradation to subvert host innate immunity [6]. Bacteria have to avoid recognition by the host immune system in order to establish a successful infection which bacterial autolysins enable the bacteriolyses of bacterial cell walls trim cell surface PGN to prevent detection by bacterial innate immune system [7].
Viruses are obligate intracellular parasites that cause infection by invading cells of the body. Their life cycle comprises a short extracellular period and a longer intracellular period during which they undergo replication. The immune system has non-specific and specific mechanism that attack the virus in both phases of its life cycle which specific antibodies protect against viral infections and play an important role in antiviral immunity, mainly during the early stage of the infection [8].
Zinc homeostasis during acute phase response is the temporal transfer of serum zinc to the tissues, causing transient serum hypokinemia, which is rebalanced during resolution of the inflammatory response that intracellularly increased zinc can intoxicate engulfed pathogens and acts cytoprotective by promotion of neutralizing reactive oxygen species (ROS) and nitrogen species (RNS) [9].
In this review, firstly, anti-bacterial activities of bacteriolysis by Zn2+ ions induced autolytic PGN activation are debated against Staphy-lococcus aureus (S. aureus) cell wall as Gram-positive bacterium and Escherichia coli(E. coli) cell wall as Gram-negative bacterium. Secondly, the zinc-mediated antiviral immunity, zinc-finger protein, membrane fusion protein and phage endolysin are discussed. Lastly, the bacterial and virucidal mechanisms on the zinc-binding activated PGN autolysins and on the ZAP, ZBD become clarified.
Zn2+ ions-induced PGN autolysins promote anti-bacterial activityMolecular structures of S. aureus and E. coli cell walls and action sites of PGN autolysins
Bacterial PGN structure of both Gram-positive and Gram-negative bacteria comprises repeating disaccharide backbones of N-acetylglucosamine (NAG) and β-(1-4)-N-acetylmuramic acid (NAM) that are crosslinked by peptide stem chains attached to the NAM residues [10]. As shown in (Figure 1), the action sites of bacterial autolysins are comprised that for Staphylococcus aureus (S. aureus) PGN layer cell wall, there are N-acetylmuramidase-L-alanine amidase and DD-endopeptidase. The other, for Escherichia coli (E. coli) cell wall as shown (Figure 2), there are endopeptidase of degrading enzyme at lipoprotein of C- and N-terminals, and amidase, peptidase, and caboxypeptidase at thin PGN layer in periplasmic space [11]. The bacterial cell walls are a strong flexible mesh work of PGN that gives a bacterium structural integrity, in which to accommodate a growing cell, the walls are remodeled by PGN synthesis and PGN autolysin. PGN is the main constituent of bacterial cell walls and must be continuously synthesized and degraded to maintain the integrity and viability of the cells that bacterial cell wall hydrolases of amidase,glycosidase, and peptidase display a modular architecture combining multiple and different catalytic domains, including some lytic transglycosy-lases as well as cell wall binding domains [12]. In these autolysins, zinc-dependent PGN autolysin of amidases may be enhanced and induced anti-bacterial vaccine activities.
Zn2+ ions induced activated PGN autolysins promote anti-bacterial activity against Gram-positive bacteria:
S. aureus amidase AmiA shed light on PGN binding and cleavage that amiA distinguishes PGN mostly by the peptide, and cleavage is facilitated by a zinc-activated water molecule, in order to develop new therapeutics against MRSA [13].
The autolytic activity of the recombinant amidase of the Aas (autolysin/adhesin of Staphylococcus saprophyticus) is inhibited and is neccesary for the C-terminal GW repeats, not the N-terminal repeats [14]. Autolysin-mediated lysis-induced bacterial cell death can contribute to the bactericidal vaccine activities. Lytic amidase autolysin LytA which is released by bacterial lysis, associates with the cell wall via its zinc-binding motif that the amidase domain comprises a complex substrate-binding crevice and needs to interact with a large-motif epitope of PGN for catalysis [15]. Suicidal amidase autolysin LytA having both autolysis and capsule shedding depends on the cell wall hydrolytic activity of LytA that capsule shedding drastically increases invasion of epithelial cells and is the main pathway by which pneumococci reduce surface bound capsule during early acute lung infection of mice [16]. In the biofilms increase as zinc concentrations increase and biofilm formation effect as a negative regulator of LytA dependent autolysis, zinc availability contributes to the ability of pneumococci to form aggregates and subsequently, biofilms [17]. The LytB PGN hydrolase responsible for physical separation of daughter cells cleaves the GlcNAc-β-(1,4)-MurNAcglycosidic bond of PGN building units that cell walls digestion products and solubilisation rates might indicate a tight control of LytB activity to prevent unrestrained breakdown of the cell wall [18]. The PGN-remodeling autolysins LytC, LytD, and LytF are expressed in the same subpopulation of cells and complete flagella synthesis that LytC appears to be important for flagella function, motility was restored to a LytC mutant by mutation oef either lon A, and LytC, LytD, andLytF autolysins to population heterogeneity in B. subtilis[19]. Atl is the major autolysin in S aureus that the bifunctional major autolysin plays a key role in staphylococcal cell separation which processing of Atl yield catalytically active amidase and glucosidase domains [20]. The biochemical and strucural staphylococcal Atl have successful cloaning, high level over-expression, and purification Atl proteins [21]. Major Atl autolysin also have an essential role in the early events of the fibronectin-binding proteins (FnBPs)-dependent S.aureus biofilm phenotype [22]. For the contribution of autolysins of PGN hydrolases to bacterial killing, there are N-acetylglucosaminidase (AtlA), two N-acetyl-muraminases (AtlB and AtlC) [23]. AtlA is the major PGN hydro-lases of Enterrococcusfaecalis involved in cell division and cellular autolysis and the zinc metalloprotease, gelatinase (GelE) of their interplay proposed to regulate AtlA function, which N-terminal cleavage was required for efficient AtlA-mediated cell division, and AtlA septum localization and subsequent cell separation can be modulated by a single GelE-mediated N-terminal cleavage event [24].
Zn2+ ions induced degrading enzyme of outer membrane lipoprotein and PGN autolysins promote anti-bacterial activityagainst Gram-negative bacteria against Gram-negative bacteria
Amidase gene (AmiB) catalyzes the degradation of PGN in bacteria that the amiB gene was composed of 1,722 nucleotides and 573 amino acids which is involved in the separation of daughter cells after cell devision and inactivation of the amiB gene, resulting in a marked increases of sensitivity to oxidative stress and organic acids [25]. Amidase activity of amiC controls cell separation and PGN fragments release [26]. Zinc-dependent endopeptidases (Eps) are predicted to hydrolyze PGN to facilitate cell growth that zinc avaliability affects strong activity of cell wall hydrolases, and zur-regulated endopeptidases are present in divergent Gram-negative bacteria [27]. Zinc-regulated peptidase maintains cell wall integrity during immune-mediated nutrient sequestration against Acinetobacterbaumannii [28].
Carboxypeptidases are exopeptidases that remove a single amino acid residue from the C terminus of proteins or xopeptidases that remove a single amino acid residue from the C terminus of proteins or peptides that the carboxypeptidase B1 of and its evaluation have been high molecular characterization for transmission-blocking vaccines (TBVs) against Malaria eradication [29]. Metallocarboxypeptidases (MCPs) of the M32 family of peptidases exhibit a significant hydrolytic activity and different hydrolysis patterns against Trypanosomabrucei or cruzi [30]. Thus, zinc-dependent carboxypeptidase autolysin could adapt to be appreciable the anti-bacterial activities.Table 1 represents anti-bacterial activities of bacteriolysis by Zn2+ ions induced activated PGN autolysins against Gram-positive thick PGN layer and Gram-negative outer membrane lipoprotein and thin PGN layer cell walls.
NAnti-viral activities on zinc-induced antiviral immunity,zinc-finger protein, zinc-binding domain, andmembrane fusion protein
Zinc-induced antiviral immunity: Zinc is an essential trace element that is crucial for growth, development, and the maintenance of immune function whichzinc status is a critical factor that can influence antiviral immunity, particularly as zinc-deficient populations are often most at risk of acquiring viral infections such as HIV, HCV [31]. Commonfeatures possess that enveloped viruses enter cells by membrane-fusion protein on the surface, fusion glycoprotein on metastable prefusion and interactions with neutralizing antibodies. Implications for immunogenic design of next-generation vaccines have been shown from the results that stable immunogens presenting the same antigenetic sites as the labile wild-typeproteins efficiently elicit potently neutralizing antibodies [32].
Zinc-finger protein: The novel EBV-induced zinc finger gene, ZNFEB, including its intron less locus and human protein variants, controls entry andexit from cell cycling in activated lymphocytes [33]. The designed polydactyl zinc finger protein is prepared consisting HIV-1 type integrase fused to the synthetic zinc finger protein E2C that the integrase-E2C fusion proteins offer an efficient approach and a versatile framework for directing the integration of retroviral DNA into a predetermined DNA site [34]. Artificial zinc finger fusions were targeted to the high affinity Sp1-binding site, and by being fused with TatdMt and POZ domain, they strongly block both Sp1-cyclin T1-dependent transcription and Tat-dependent transcription of HIV-1 [35]. The zinc-finger antiviral protein (ZAP) specifically inhibits the replication of certain viruses and promotes viral RNA degradation [36]. Zinc finger protein Tsip1 that the candidate genes encoded Tsi1?interacting protein 1 (Tsip1), a zinc (Zn) finger protein Tsip1 strongly interacted with CMV 2a protein, controls Cucumber mosaic virus (CMV) RNA replication [37]. The zinc-finger protein ZCCHC3 binds RNA and facilitates viral RNA that ZCCHC3 is a co-receptor for the retinoic acid-inducible gene-1 (RIG-1) and antigen MDA5 which is critical for RIG-1 like receptor (RLR)-mediated innate immune response to RNA virus [38].
Zinc-binding domain:A novel zinc-binding domain (ZBD) is essential for formation of the functional Junin virus envelope glycoprotein complex that the envelope glycoprotein of the Junin arenavirus (GP-C) mediates entry into target cells through a pH-dependent membrane fusion mechanism, in which this unusual motif may act to retain a cleaved 58-amino-acid stable signal peptide (SSP) for its role in modulating membrane fusion activity [39]. Entry of the virus into the host cell is mediated by the viral envelope glycoprotein, GPC that SSP was retained in GPC through interaction with a zinc- binding domain (ZBD) in the cytoplasmic tail of transmembrane fusion of G2 subunits that Junin virus ZBD displays a novel fold containing two zinc ions, in which the structural basis for retention of the unique SSP submit suggests a mechanism whereby SSP is positioned in the GPC complex to modulate pH-dependent membrane fusion [40].
Viral membrane fusion protein: Enveloped viruses enter cells and initiate disease-causing cycles of replication that in all cases virus-cell fusion is executed by one or more viral surface glycoproteins denoted as the fusion protein, in which the structure and mechanisms on viral membrane fusion protein are important problems [41]. The membrane fusion reaction, membrane interaction, conformational changes of specialized virus envelope proteins, and refolding reactions of specific fusion proteins can mediate both virus-cell fusion leading to infection and pathological cell-cell fusion, in which they are increasingly viewed as targets for antiviral intervention [41].
Phage endolysin: Bacteriophage (phage) is a virus that precisely infects bacterial hosts that after the completion of a replication inside the infected bacterial cell, newly formed phage particles need to be released outside the cell with the help of lytic enzymes, these lytic enzymes of endolysin, in which endolysins are bacteriophage-encoded peptidoglycan hydrolase [42]. Phage endolysin of cell wall binding domains (CBDs) is characterized in conjuction with their domain architecture, (non)necessity for the following lytic activity and a high/low specificity of their ligands as well [42]. Thus, anti-viral activity of zinc-finger proteins for virus entry and replication are represented in Table 2.
Conclusion
Anti-infectious activities of bacteriolyses by Zn2+ ions induced activated PGN autolysins and of virucides by zinc-binding viral fusion proteins are discussed, and the bacteriolytic and virucidal mechanisms are partially clarified.Bacterial peptidoglycan (PGN) autolysin AmiA for S.aureus amidase is acted on PGN binding and cleavage that amiA distinguishes PGN mostly by the peptide, and cleavage is facilitated by a zinc-activated molecule. The autolytic activity of the recombinant amidase of the Aas (autolysin/adhesin of Staphy-lococcussaprophyticus) is inhibited and is necessary for the C-terminal GW repeats, not the N-terminal repeats. AmiB catalyzes the degradation of PGN in bacteria, resulting in a marked increases of sensitivity to oxidative stress and organic acids. Amidase activity of amiC controls cell separation and PGN fragments release. In these autolysins, zinc-dependent PGN autolysin of amidases may be enhanced and induced anti-bacterial activities.
Lytic amidase autolysin LytA associates with the cell wall via its zinc-binding motif.The LytB PGN hydrolase responsible for physical separation of daughter cells cleaves the GlcNAc-β-(1,4)-MurNAcglycosidic bond of PGN building units. LytC, LytD, and LytF are expressed in the same subpopulation of cells and complete flagellar synthesis. Human peptidoglycan recognition proteins (PGLYRPs) are novel class of recognition and effector molecules with broad Zn2+-dependent bactericidal activity against both Gram-positive and Gram-negative bacteria.
Enter toxigenic E.coli (ETEC) is the most common bacterial cause of children's diarrhea, in which antigen and antitoxin antibodies that neutralized both toxins that are associated with all cases of ETEC diarrhea. Thus, Autolysin mediated bacteriolysis-induced bacterial cell death can contribute to the bactericidal activities. Bacterial autolysins enable the bacteriolyses of bacterial cell walls trim cell surface PGN to prevent detection by bacterial innate immune system. Autolysin mediated bacteriolysis and zinc dependent lysis-induced bacterial cell death can contribute to the bactericidal vaccine activities, where PGN autolysins interact with biomolecules causing cell apoptosis leading to cell death.
On the other hand, enveloped viruses enter cells and initiate disease-causing cycles of replication that in all cases virus-cell fusion is executed by one or more viral surface glycoproteins denoted as the fusion protein, in which the structure and mechanisms on viral membrane fusion protein are important problems. The novel EBV-induced zinc finger gene, ZNFEB, including its intron less locus and human protein variants, controls entry and exit from cell cycling in activated lymphocytes.The designed polydactyl zinc finger protein is prepared consisting HIV-1 type integrase fused to the synthetic zinc finger protein E2C that the integrase-E2C fusion proteins offer an efficient approach and a versatile framework for directing the integration of retroviral DNA into a predetermined DNA site. The zinc-finger antiviral protein (ZAP) specifically inhibits the replication of certain viruses and furthermore,an under-standing becomes necessary for ZAP-mediated viral RNA degradation. Zinc finger protein Tsip1 controls Cucumber mosaic virus (CMV) RNA replication. The zinc-finger protein ZCCHC3 binds RNA and facilitates viral RNA that ZCCHC3 is a co-receptor for the retinoic acid-inducible gene-1 (RIG-1) and MDA5 which is critical for RIG-1 like receptor (RLR)-mediated innate immune response to RNA virus. Zinc-finger protein, zinc-binding domain, and membrane fusion protein specifically inhibit the entry and the replication of many viruses. Thus, the membrane fusion reaction, membrane interaction, conformational changes of specialized virus envelope proteins, and refolding reactions of specific fusion proteins an essential steps entry and replication of enveloped virus life cycle have been worthy of remark in fascination that these diverse viral fusion proteins could be used in next-generation for therapeutic intervention in arena viral disease.
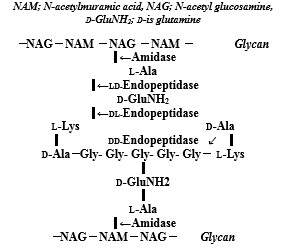
Figure 1: Peptidoglycan structure and action sites of peptidoglycan autolysins against S. aureus PGN layer.
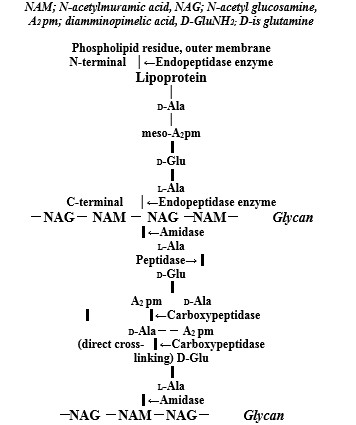
Figure 2: Molecular structures of outer membrane lipoprotein and peptidoglycan layer in the E. coli cell wall, and action sites of degrading enzyme oflipoprotein at C- and N-terminals and peptidoglycan autolysins
|
Zn2+ Ions |
Gram-Positive PGN Layer Cell Wall |
|
|
Zn2+ |
Zn2+ ions induced PGN autolysins → Zn2+, O2?, H2O2, ?OH, ?NO, ONOO? Zn2+ ions induced activated PGN autolysins ?S.aureusamidase AmiA ?Recombinant amidase of the Aas ?Lytic amidase LytA for Streptococcuspneumoniae ?Pneumococcal autolysinLytALytC, D, F of PGN remodeling for Bacillus subtilis ?Endopeptidase LytF for bacillus subtilis ?AtlA autolysin for GelE against E. faecalis ?AtlA, AtlB, AtlC autolysins against enterococcus faecalis ?Fusion protein autolysin, MIBRs against S. pneumoniae ?Carboxypeptidase B1 against Anopheles stephensiand for malaria as transmission blocking vaccines ?Metallocarboxypeptidase M32 against Trypanosomabruceior cruzi ?PBP2a and autolysin mixture against MRSA |
|
|
Zn2+ ions |
Gram-Negative Cell Wall |
|
|
Zn2+ |
Outer Membrane Lipoprotein |
Periplasmic Space Thin PGN |
|
at C- and N-terminals |
Layer |
|
|
→ Zn2+, O2?, H2O2 |
→ Zn2+, O2?, H2O2, OH?, ?OH |
|
|
?Amidase gene amiB/LysM |
?AmiC in PGN fragment release |
|
|
?Endopeptidase regulation of ShyA and ShyB |
?Carboxypeptidase by transmission- |
|
|
?Outer membrane receptor against N.menigitidis |
blocking vaccines |
|
|
?ETEC subunit vaccine |
?PGRPs or PGLYRPs |
|
|
?ZnuB against P. aeruginosa. |
?D-glutamate auxotrophy against P. |
|
|
?Preventive vaccine by recombinant flagella against P. aeruginosa |
aeruginosa PA14 |
|
|
?ORT in infectious diarrhoea |
||
|
?ZnuA against P. aeruginosa |
||
|
?Recombinant flagella and pili against P.aeruginosa |
||
Table 1:Zinc induced anti-bacterial activity against Gram-positive thick PGN envelope cell wall and Gram-negative lipoprotein and thin PGN layer cell wall.
|
Zn2+ ions |
Anti-viral activity of Zn2+ in entry and replication |
|
|
Adsorption/Entry |
Replication, DNA / RNA |
|
|
Zn2+ |
→ Zn2+, ?O2-, H2O2 |
→ Zn2+, ?O2-, H2O2, NO |
|
?EBV-induced zinc finger gene ZNFEB controls entry and exit |
?ZAP inhibits replication of MLV |
|
|
?ZAP-mediated RNA degradation |
||
|
?ZBD prevent viral entry and and GPC inhibit activate membrane fusion |
?Zinc finger: virus decay |
|
|
?Zinc finger proteinE2C; viral DNA specific sites |
||
|
?Zn-metalloprotease inhibits entry and cell-cell fusion
|
?Zinc finger protein Tsip1;Cucumber mosaic virus(CMV)RNA replication |
|
|
?Artificial zinc finger fusion; HIV-1 transcriptions |
||
Table 2: Anti-viral activity of zinc-finger proteins for virus entry and replication.
Citation: Ishida's T (2019) Anti-Infectious Activities for Bacterial Zn2+-Induced Peptidoglycan Autolysins and Viral Zinc-Finger Fusion Proteins against Bacterial and Viral Infections. Ann Med & Surg Case Rep: AMSCR-1000028.