Open Access Journal of Agriculture Research
(ISSN 2651-9003)
Research Article
Haematotoxic and Genotoxic Potential of Penconazole and Copper Nanoparticles on Redbelly Tilapia (Tilapia Zillii)
Osman AGM1,2*,Elmileegy IMH3, Farrag MMS1,Said RE1, Khalil NSA3, El-Sawy MF1 and Ahmed ES4
1Department of Zoology, Al-Azhar University, Egypt
2Department of Ecophysiology and Aquaculture, Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Germany
3Department of Medical Physiology, Assiut University, Egypt
4Department of Veterinary Physiology, Assiut University, Egypt
*Corresponding author:Alaa G.M. Osman, Department of Zoology, Al-Azhar University, Assiut, Egypt, Tel: 00201126580911; Email: agosman@azhar.edu.eg
Citation: Osman AGM, Elmileegy IMH, Farrag MMS, Said RE, et al. (2019) Haematotoxic and Genotoxic Potential of Penconazole and Copper Nanoparticles On Redbelly Tilapia (Tilapia Zillii). Open Acc J Agri Res: OAJAR-100019
Received date:26 September, 2019;Accepted date:03October, 2019; Published date:21 October, 2019
Abstract
The extensive use of fungicides in both conventional and nanoengineered forms poses a major health threat to aquatic organisms. Some of the most sensitive and valid biomonitoring parameters for assessment of aquaculture toxins are changes in haemato-genotoxic endpoints. Therefore, this study aimed to examine the effects of penconazole (PEN) and copper nanoparticle (Cu-NP) fungicides on haematological parameters and erythrocyte micronucleus (MN) and nuclear abnormalities (ENAs) formation in male and female of Tilapia zillii and to elucidate differences with respect to dose and sex. A total of 180 normal adult Tilapia zillii were allocated equally to five groups with three replicates of 12 fish. The control group received no treatment, the PEN (I) group was exposed to PEN at a dose of 0.8 µg/L, the PEN (II) group was exposed to PEN at a dose of 1.6 µg/L, the Cu-NP (I) group was exposed to Cu-NP at a dose of 7.5 mg/L, and the Cu-NP (II) group was exposed to Cu-NP at a dose of 15 mg/L. The results showed that PEN and Cu-NPs markedly reduced RBC counts; induced leucocytosis, thrombocytosis and lymphocytosis; and obviously increased the percentages of MNs and ENAs in a dose-dependent manner. Males and females exhibited differential sensitivity to PEN and Cu-NPs depending on the dose and type of exposure. These patterns of adverse effects on haematological variables and the results of MN tests can be used as references for investigation of the ecotoxicity of these fungicides in fish. Further studies are highly recommended to explore the possible contributory factors involved in the haemato-genotoxic effects of these aquatic contaminants.
Key words:Copper nanoparticles; Haematology; Penconazole; Tilapia zillii
Introduction
The redbelly tilapia(Tilapia zillii) is a native fish in Egypt [1]. However, its adaptability to variable environmental conditions along with its rapid growth rate, high fecundity, and omnivorous feeding habits has allowed this species to breed and establish itself in areas outside its native range[2]
Exposure of fish to fungicides is a major ecotoxicological concern because of the broad application of these chemicals in humans and domesticated animals [3]. Thus, it is necessary to evaluate the impacts of fungicide exposure on a laboratory scale in order to investigate in depth the effects of these chemicals on multiple physiological systems without possible interactions with other aquatic toxicants. Studying the effects of fungicides on fish may provide valuable information, suggesting new research directions or yielding evidence to support the pathological signs that are suspected in humans [4].
Penconazole (PEN) is one of the most commonly used triazole fungicides in many countries throughout the world [5]. Triazole fungicides enter ecosystems through spray drift or in surface run-off after rainfall [6].Its persistence in the environment, bioaccumulation through the food chain and resistance to degradation have caused much attention to be paid to the health hazards of PEN as an aquatic toxicant [7, 8].Developments over the past decade in the field of nanotechnology and agriculture have led to the commercialization of nanobased products such asNano fungicides [9]. Despite the potential advantages of nanofungicides, many issues have still not been addressed. For instance, there is a growing public debate on the haematological impact of exposure to Nano formulated fungicides [10].
Blood cells are some of the first cells to come into contact with aquatic pollutants; in fish, measurable physiological changes occur more rapidly in these cells than in any other physiological assessment parameters [11]. Therefore, assessment of haematological endpoints has been suggested to be a simple and compelling method for understanding disturbances in physiological processes and for rapidly assessing health status in fish exposed to fungicides [12]. Changes in differential leucocyte counts are recognized as sensitive indicators ofenvironmental stress responses [13]. In addition, blood contains reliable biomarkers for detection of damage at the level of DNA [14]. Several techniques have been developed for direct detection of DNA damage, including assays for assessment of the frequencies of micronuclei (MNs) and other erythrocyte nuclear abnormalities (ENAs). These techniques are the most widely applied methods since they allow for convenient and easy application, particularly in genotoxicological studies on fish[15-17]. MN assays are valuable and sensitive tools for detection of genotoxicity caused by agricultural fungicides in surface run-off and for in situ monitoring of water quality[18]. Genotoxic effects in fish are a matter of great concern because of the potential risks to human health after consumption [16].
A few studies have addressed the haematological effects of conventional and nanobased fungicides. For example, exposure of rainbow trout and Indian major carp to propiconazole (PCZ) causes macrocytic anaemia[19,20]. Similarly, copper nanoparticles (Cu-NPs) negatively affect haematological indices in juvenile Oncorhynchus mykiss[21]. However, to our knowledge, there are no available data on the potential haematotoxic and genotoxic effects of PEN and Cu-NPs on Tilapia zillii. Therefore, the aim of this study was to elucidate these effects and their dependence on dose and sex. This work is of significance from an ecotoxicological perspective and paves the way for further research on the potential molecular mechanisms underlying the effects of these pollutants.
Materials and methods
Chemicals
PEN was purchased from Zhejiang Hisun Chemical Company, Ltd., China. Cu-NPs were purchased from Shield Plant Company, Egypt. All chemicals used for haematological and MN tests were purchased from Alpha Chemicals Company and El-Nasr Pharmaceutical Chemicals Company, Egypt.
Determination of the LC50 values of PEN and Cu-NPs
Pre-acclimatized Tilapia zillii were held in 6 glass aquaria (25 litre capacity), each of which contained six tilapias, representing one group.Feeding was stopped 24 hours before the commencement of the test, and food was withheld during the experimental period to prevent adsorption of nanoparticles (NPs) to food or faecal material [22].The dead fish were counted every 12 hours and removed immediately to prevent contamination of the exposure solution. For determination of the median lethal concentration (LC50) of PEN, a static renewal acute toxicity bioassay was conducted according to Organisation for Economic Cooperation and Development (OECD) Directive No. 203, a fish acute toxicity test[23]. The fungicides were dissolved in 100 mL of distilled water and then added to the aquaria. The control group received 100 mL of distilled water. The other groups were exposed separately to single doses of PEN (10 µg/L, 12 µg/L, 14 µg/L, 16 µg/L, 18 µg and 20 µg/L) that were renewed daily. The 96 hour LC50 was calculated according to the following equation:
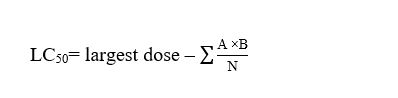
where A is the dose difference between two successive doses, B is the mean number of dead fish between two successive doses, and N is the total number of fish per group.
Next, fish were exposed to 1/10 of the LC50 (1.6 μg/L) and 1/20 of the LC50 (0.8 μg/L) of PEN for 3 months. The LC50 of Cu-NPs was 150 mg/L (1/10 LC50=15 mg/L and 1/20 LC50=7.5 mg/L) [24].
Fish maintenance
A total of 180 normal adult Tilapia zilli of both sexes with an average body weight of 20-40 grams and an average length of 10-18 centimetres were collected from the river Nile at Assiut, Egypt during 2017. The fish were considered normal based on their external appearances and the absence any disease symptoms. The fish were transported to the fish biology laboratory at the Zoology Department, Faculty of Science, Al-Azhar University, Assiut Branch, and kept in 160 litre rectangular tanks containing dechlorinated tap water (conductivity, 2000 µs/cm; pH, ~7.5; oxygen saturation, 90-95%; temperature, 25 °C; and photoperiod, 12 h:12 h light: dark). The fish were acclimated for 21 days. During this period, the fish were fed commercial feed pellets (40% crude protein, 4.22% fat, 5.88% crude fibre, 10.30% ash, and 10.03% moisture) at a rate of 3% of their body weight per day in two feedings, and the water was renewed every two days.
Experimental groups and sampling
After 21 days of acclimatization, fish of both sexes (90 males and 90 females) were equally and randomly divided into five groups with three replicates of 12 fish. Group one served as the control group; group two, the PEN (I) group, was exposed to PEN at a dose of 0.8 µg/L; group three, the PEN (II) group, was exposed to PEN at a dose of 1.6 µg/L; group four, the Cu-NP (I) group, was exposed to Cu-NP at a dose of 7.5 mg/L; and group five, the Cu-NP (II) group, was exposed to Cu-NP at a dose of 15 mg/L. Males and females were placed together in the same tanks. The tanks were aerated with air stones attached to an air compressor to ensure oxygen saturation.
At the end of the experiment, which lasted for 3 months, 9 fish per group were randomly collected and anaesthetized with a 0.02% benzocaine solution. Two blood samples were collected from the caudal vein according to the methods of[24]. The first blood sample was collected in a heparinized tube for quantification of red blood cells (RBCs), white blood cells (WBCs) and platelets by the haemocytometer method. The second blood sample was used for analysis of MN and ENA formation as described previously[25]. The blood samples were smeared on clean microscope slides. After fixation in pure methanol for 20 minutes, the slides were air-dried and stained with 5% Giemsa solution for 30 minutes. Ten slides per treatment were prepared from each animal, and 1000 cells were scored under 1000 ×magnification to determine the frequencies of MNs and binucleated erythrocytes. The coded and randomized slides were scored blindly by a single observer.
Scoring criteria for MNs and nuclear lesions
Only cells that were clearly isolated from the surrounding cells were scored. In accordance with the methods of[26], the criteria for the identification of MNs were as follows: (a) MNs had to be smaller than one-third the size of the main nuclei, (b) MNs had to be clearly separated from the main nuclei and (c) MNs had to be on the same plane of focus and have the same colour. Cells with more than four MNs were discarded to exclude apoptotic phenomena. Binucleated erythrocytes were defined as those with nearly two equal-sized nuclei. Nuclei with vacuoles and nuclei in which nuclear material could not be detected after examination to an appreciable depth were recorded as notched nuclei. Kidney- and heart-shaped nuclei both lacked nuclear material upon examination to an appreciable depth. Irregularly shaped nuclei were also noted.
Statistical analysis
The data are represented as the mean ± standard error of the mean (SEM). The results were analysed by one-way analysis of variance (ANOVA) followed by Duncan’s post-test using SPSS version 16 (SPSS Inc., Chicago, USA). Differences of P<0.05 were considered statistically significant.
Ethical statement
This experimental study was performed according to the guidelines of the research ethics committee of the Faculty of Science, Al-Azhar University, Assiut Branch.
Results
Effects of the different treatments on RBC, WBC, and platelet counts
Table 1 shows the changes in the RBC, WBC and platelet countsfollowing exposure to the fungicides PEN and CU-NPs. Exposure of female tilapia to PEN at doses of 0.8 and 1.6 μg/L resulted in significant reductions in RBC counts in the treated groups compared with the control group. On the other hand, in males, the RBC counts were significantly lower in all treated groups than in the control group.
Among males, leucocytosis was observed in the PEN (I), Cu-NP (I), and Cu-NP (II) groups compared to the control group; in contrast, among females, leucocytosis was observed only in the PEN (I) and Cu-NP (II) groups compared to the control group. Thrombocytosis was clearly present in both males and females in the PEN (I), PEN (II) and Cu-NP (II) groups.
Effects of the different treatments on differential leucocyte counts
Effects of the different treatments on the percentage of MNs and ENAs
Table 3 demonstrates the genotoxic effects of PEN and Cu-NP on erythrocytes, as evidenced by significant increases in the percentages of MNs in females in all exposed groups and in males in all groups except the Cu-NP (II) group compared to the control group. The percentages of notched nuclei and binucleated erythrocytes were significantly increased in the PEN (I) and PEN (II) groups for males and in the PEN (II) group for females. The percentage of heart-shaped nuclei was significantly increased only in the PEN (I) group for males, whereas it was significantly increased in both the PEN (I) and PEN (II) groups for females. Among males, the percentage of kidney-shaped nuclei was significantly increased in all exposed groups except the Cu-NP (II) group. Among females, the percentage of kidney-shaped nuclei was significantly increased only in the PEN (I) and PEN (II) groups. The percentage of irregularly shaped nuclei was significantly increased in the PEN (II) group for males, while it was significantly increased in all exposure groups except the Cu-NP (I) group for females. The various forms of ENAs are shown in Figure 1.
Discussion
To our knowledge, this study is the first assessment of the effects of the fungicides PEN and Cu-NPs on the haematological parameters of Tilapia zilli. In this study, we observed clear haematotoxic effects of PEN and Cu-NP in both male and female Tilapia zilli given the obvious cytotoxicity in blood cells and genotoxicity in erythrocytes.
Based on the findings of this study, patterns of alteration in haematological parameters can be used as potential biomarkers for evaluation of the toxicity of the examined fungicides toward aquatic organisms. Our findings highlight the need for safe disposal protocols for these and other fungicides that might be released into surface waters and suggest that much attention should be paid to preventing extensive use of these fungicides in field crops and reducing surface run-off of these fungicides near water bodies. In the current study, clear reductions in RBC counts were observed in males following exposure to both types of fungicides at all doses and in females exposed to PEN at doses of 0.8 and 1.6 μg/L. This outcome is consistent with that observed in rainbow trout and Indian major carp following exposure to PCZ and in juvenile Oncorhynchus mykissfollowing exposure to Cu-NPs[27].
Inhibition of erythropoiesis, exhaustion of kidney haematopoietic activity, and elimination of RBCs from circulation may be contributory factors to the marked decreases in RBC counts[28,29,30]. Other explanations include increases in RBC haemolysis rates due to disruption of oxidant/antioxidant balance, depletion of intracellular ATP, and disturbance of elements that equilibrate the intracellular osmotic pressure[31,32,33].
The reductions in RBC counts in the PEN- and Cu-NP-challenged groups reflect the development of anaemia, which is characterized by decreased oxygen carrying capacity in the blood that prevents the circulation from supplying sufficient oxygen to the tissues [34].
Cu-NP exposure could have contributed to the reductions in RBC counts given the cytotoxic and cellular antiproliferative properties of Cu-NPs, which are secondary to their ability to induce reactive oxygen species production and cell cycle arrest [35].
WBCs are the main regulators of the immune system and help defend the body against both infectious diseases and foreign substances[36]. In this study, the observed leucocytosis in the PEN (I), Cu-NP (I) and Cu-NP (II) groups for males and in the PEN (I) and Cu-NP (II) groups for females indicated that the fungicides under investigation stimulated the immune response in Tilapia zillii. Similar findings have been obtained in Indian major carp (Labeo rohita) exposed to iron oxide NPs [37] and in rainbow trout (Oncorhynchus mykiss)exposed to clotrimazole [38]. Toxic stress caused by contaminants may stimulate lymphopoiesis and/or enhance the release of lymphocytes from lymphomyeloid tissue, leading to increases in WBC counts[39].
In the current study, exposure of both males and females to PEN at doses of 0.8 and 1.6 μg/L and to Cu-NPs at a dose of 15 mg/L caused thrombocytosis. Previously, four-week exposure of Barbus conchonius Hamilton to sublethal levels of endosulfan and phosphamidon was found to induced thrombocytosis that persisted even after recovery in clean water[40]. In addition, Labeo rohitaexposed to 10, 20, 30, 45, and 55 mg/L Ag-NP for 28 days’ show marked increases in platelet counts[41]. The significant elevations in platelet count that were observed in the present work may have been defensive haemostatic reactions to internal injuries induced by PEN and Cu-NPs after their entry into the circulatory system that subsequently increased their systemic toxic effects.
T- and B-lymphocytes, as principal components of adaptive immune mechanisms in fish, play vital roles in protection against recurrent infections[42]. In this study, lymphocytosis was evident in both males and females following exposure to PEN at doses of 0.8 and 1.6 μg/L and to Cu-NPs at a dose of 15 mg/L. This finding is consistent with changes observed in African catfish exposed to carbofuran at doses of 0.16 and 0.49 mg/L for 35 days but contradicts changes observed in Cyprinus carpioexposed to tebuconazole at a dose of 2.5 mg/L for 14 days [43] and in Oreochromis mossambicusexposed to zinc oxide NPs at doses of 30, 50 and 70 mg/L for 96 hours [44]. This contradiction may be related to differences in the design of the experiment, species of fish, duration of exposure and type and dose of fungicide. The lymphocytosis observed in the current study indicates stimulation of specific immune responses under PEN and Cu-NP challenge as protective mechanisms against the contaminants.
Neutrophils, the main cells involved in phagocytosis in fish [45], remove bacteria by producing reactive oxygen species during respiratory bursts. In addition, neutrophils possess myeloperoxidase in their cytoplasmic granules, which kills bacteria in the presence of halide and hydrogen peroxide through halogenation of the bacterial cell wall. Moreover, these cells have hydrolytic enzymes in their lysosomes [46]. In the present work, the neutropaenia in males and females in the PEN (I), PEN (II), and Cu-NP (II) groups indicates that inhibition of the non-specific immune defence system occurred, leaving the fish more susceptible to bacterial infection. Polyacrylic acid-coated superparamagnetic iron oxide NPs activate intrinsic and extrinsic apoptotic pathways and trigger oxidative bursts in a nicotinamide adenine dinucleotide phosphate oxidase-dependent manner in human neutrophils [47]. Such effects may explain the marked reductions in neutrophil counts in our study following exposure to the Cu-NPs.MNs and other ENAs, which are widely used as indicators of exposure to genotoxic and mutagenic contaminants in fish species, have been assessed as possible additions to the necropsy-based, histopathological, serological and molecular indicators currently utilized. High incidences of MNs have been observed in fish peripheral erythrocytes after exposure to different pollutants under field and laboratory conditions[48,49,50]. The results of the present study showed obvious increases in the percentages of MNs and of notched, kidney-shaped and binucleated nuclei in males and in the percentages of heart-, kidney- and irregularly shaped nuclei in females following exposure to PEN at doses of 0.8 and 1.6 μg/L. Exposure of females to PEN at a dose of 1.6 mg/L markedly increased in the percentages of notched and binucleated nuclei, while exposure of males to PEN at a dose of 0.8 mg/L markedly increased the percentage of heart-shaped nuclei. In a previous work, exposure of walking catfish (Clarias batrachus) to 1.11 and 2.23 mg/L PCZ for 96 hours induced formation of MNs and other nuclear anomalies, including notched nuclei, nuclear buds and nucleoplasmic bridges, in peripheral erythrocytes. Nitrogen-containing substances, including main PCZ metabolites, induce the production of reactive oxidants that adversely impact the nucleic acids in fish blood [51].
An MN forms during telophase of cell division when either whole or fragmented chromosomes become encapsulated in a nuclear envelope and assume the properties of an interphase nucleus that is dramatically reduced in size [52]. MNs originate from lagging acentric chromosome or chromatid fragments caused by misrepair or lack of repair of DNA breaks or by anaphase malsegregation of whole chromosomes. Malsegregation of whole chromosomes results from hypomethylation of repeat sequences in centromeric and pericentromeric DNA and defects in kinetochore proteins or assembly, spindle fibres, and anaphase checkpoint genes [53]. Regression of cleavage furrows, failure of cytokinesis, formation of multipolar spindles and merging of newly formed cells are considered to be the most important causative factors responsible for the appearance of binucleated cells [54]. It has been suggested that gene amplification via the breakage-fusion-bridge cycle causes the formation of nuclear anomalies during elimination of amplified DNA from the nucleus [55].
In the current experimental model, exposure to Cu-NPs at a dose of 7.5 mg/L clearly increased the percentages of MNs in females and the percentages of MNs and kidney-shaped nuclei in males. In addition, marked increases in the percentages of MNs and irregularly shaped nuclei were observed in females exposed to Cu-NPs at a dose of 15 mg/L. Similarly,in a previous study, significant elevations in MNs and other ENAs were observed in Oreochromis niloticus exposed to a low concentration of copper oxide NPs (1/10 of the LC50) for 30 days, although decreases in the extent of chromosomal damage were observed after exposure to a higher concentration (1/20 of the LC50) for 30 days [56]. Another study evaluated DNA damage in adult Danio reriowith comet assays and MN tests following exposure to perfluorooctanesulfonic acid, zinc NPs or perfluorooctanesulfonic acid in combination with zinc NPs for 30 days and found that perfluorooctanesulfonic acid alone at all doses could strongly induce DNA damage in peripheral blood cells and that zinc oxide NPs could aggravate the genotoxicity in the co-treatment groups [57].
Metal NPs can enter a cell and subsequently the nucleus through diffusion across the nuclear membrane or transport through nuclear pore complexes; alternatively, they can enter nuclei following mitosis as the nuclear envelope disappears during cell division and then reforms in each daughter cell. Intranuclear metal NPs can cause chromosomal breakage or malfunction of the mitotic spindle, resulting in the formation of one or more MNs per cell [58].
Several studies have shown that the genotoxicity of copper oxide and silver NPs is due not only to the liberation of metal ions from the particles but also to the influences of particle properties (e.g., particle size, reactivity, and surface charge). Most NPs gain toxicological properties from their high surface area-to-volume ratios, which increase their reactivity and catalytic potency [59]. Furthermore, it has been proposed that the main cause of genotoxicity following exposure to many types of NPs is overproduction of reactive oxygen species, which has been found to trigger DNA strand breaks, point mutations, oxidative DNA adduct formation, and chromosomal fragmentation. Another important cause of DNA damage is direct interaction of the NPs with DNA strands [60]. After mitosis, the nuclear membrane is rebuilt; however, some NPs mayremain inside the nucleus, where they can contact DNA.NPs inside the nucleus may cause chromosomal damage due to failure of the DNA repair system and/or through physical attachment to the mitotic apparatus.
Sharp differences were observed between the sexes with regard to the effects of the fungicides on RBC counts in Tilapia zillii. A possible role of sex hormones in modulating haemolytic propensity has been suggested [61]. Compared to those in females, RBCs in males may be more susceptible to haemolysis in response to osmotic and oxidative stress due to the ability of testosterone to stimulate reactive oxygen species generation and depress resistance to free radical attack; alternatively, the differences between sexes may be due to differences in the proportions of unsaturated and saturated fatty acids in cell membranes [62,63]. On the other hand, several studies have supported the hypothesis that female sex hormones may increase membrane fluidity [64,65] which, in the case of nucleated RBCs, is likely to be mediated via genomic mechanisms.
A clear differential sex response was also verified for MN induction, as peripheral RBCsin females exhibited higher susceptibility than those in males to cytogenetic damage caused by both types of fungicides studied. The frequencies of MNs and other nuclear abnormalities have also been found to increase in Oreochromis niloticusand Dicentrarchus labraxfollowing exposure to 17β-oestradiol [66,67]. A large body of circumstantial evidence supports a predisposing role of oestrogens in genotoxic susceptibility as a consequence of their involvement in repair-induced strand breaks, improper chromosomal segregation, induction of centrosome amplification, and interference with kinase signalling that controls the spindle checkpoint [68,69]. Oestrogen metabolites also induce mutation by triggering oxidative damage to DNA [70].
Conclusion
PEN and Cu-NPs induced haematotoxicity in male and female Tilapia zillii. However, males and females exhibited differential sensitivity according to the type and dose of fungicides, confirming that female hormonal status influences haematotoxic and genotoxic responses [71]. Our results confirm that MN tests and ENA assays are sensitive enough to reveal significant differences between male and female Tilapia zillii in populations living in ecosystems with different pollution levels. Further studies are highly recommended to assess the physiological statuses of Tilapia zilli in contaminated aquaculture waters under actual field conditions because the more complex mixtures of pollutants in the environment might interfere with the outcomes.

Figure 1: Photomicrographs of erythrocytes of Tilapia zilli exposed to PEN and Cu-NP; micronuclei (A), heart shaped nucleus (B), irregular nuclei (C), notched nuclei (D), kidney shaped nuclei (E), and binucleated nuclei (G).
|
Sex |
Group/ Parameter |
Control |
PEN (I) |
Cu-NP (I) |
PEN (II) |
Cu-NP (II) |
P value |
|
Male |
RBC count (106/μL) |
4.825 ± 0.480a |
2.325 ± 0.102c |
2.083 ± 0.220c |
2.900 ± 0.629bc |
3.617 ± 0.229b |
0 |
|
Female |
- |
4.267 ± 0.299a |
2.700 ± 0.153b |
4.033 ± 0.148a |
2.267 ± 0.567b |
3.150 ± 0.338ab |
0.004 |
|
Male |
WBC count (103/μL) |
9.000 ± 0.474c |
18.825 ± 0.191a |
14.400 ± 0.924b |
9.600 ± 1.848c |
20.200 ± 1.510a |
0 |
|
Female |
- |
9.100 ± 0.756b |
20.267 ± 1.067a |
10.667 ± 1.067b |
9.800 ± 0.2000b |
18.600 ± 2.386a |
0 |
|
Male |
Platelet count (103/μL) |
114.500 ± 9.005c |
230.000 ± 9.083a |
128.330 ± 11.666c |
180.000 ± 15.275b |
197.500 ± 10.507ab |
0 |
|
Female |
- |
103.400 ± 3.945d |
195.000 ± 10.408a |
128.330 ± 11.667cd |
170.000 ± 15.275ab |
153.333 ± 14.814bc |
0
- |
|
Note:PEN (I): Group exposed to penconazole at a dose of 0.8 μg/L. Cu-NP (I): Group exposed to copper nanoformulated fungicide at a dose of 7.5 mg/L. PEN (II): Group exposed to penconazole at a dose of 1.6 μg/L. Cu-NP (II): Group exposed to copper nanoformulated fungicide at a dose of 15 mg/L. RBC: red blood cell; WBC: white blood cell. a-d Different letters indicate significance differences at P<0.05 (one-way ANOVA followed by Duncan post-test). |
|||||||
Table 1:Effects of penconazole and copper Nano formulated fungicide on the RBC, WBC and platelet count.
|
Parameter |
Group/ Sex |
Control |
PEN (I) |
Cu-NP (I) |
PEN (II) |
Cu-NP (II) |
P value |
|
Neutrophils (%) |
Male |
56.330 ± 4.667a |
|
59.670 ± 0.882a |
24.330 ± 0.333c |
40.330 ± 1.453b |
0 |
|
|
31.330 ± 1.202c |
|
|
|
|
||
|
Female |
53.330 ± 3.844a |
30.670 ± 0.667c |
54.000 ± 1.732a |
21.670 ± 1.202d |
42.000 ± 1.732b |
0 |
|
|
Eosinophils (%) |
Male |
0.330 ± 0.333 |
1.670 ± 0.333 |
1.670 ± 0.333 |
1.330 ± 0.333 |
0.670 ± 0.333 |
0.057 |
|
Female |
1.000 ± 0.577 |
1.000 ± 0.000 |
1.330 ± 0.333 |
1.330 ± 0.333 |
1.330 ± 0.333 |
0.903 |
|
|
Basophils (%) |
Male |
0.670 ± 0.333 |
0.670 ± 0.333 |
1.000 ± 0.000 |
0.330 ± 0.333 |
0.330 ± 0.333 |
0.512 |
|
Female |
1.000 ± 0.000 |
0.330 ± 0.333 |
0.330 ± 0.333 |
0.670 ± 0.333 |
0.330 ± 0.333 |
0.452 |
|
|
Lymphocytes (%) |
Male |
37.670 ± 4.485d |
60.670 ± 1.202b |
32.000 ± 1.155d |
69.000 ± 0.577a |
53.330 ± 0.667c |
0 |
|
Female |
39.000 ± 3.606d |
63.000 ± 0.577b |
38.67 ± 2.404d |
70.330 ± 0.882a |
52.000 ± 1.528c |
0 |
|
|
Monocytes (%) |
Male |
5.000 ± 0.577 |
5.567 ± 0.333 |
5.670 ± 0.333 |
5.000 ± 0.577 |
5.330 ± 0.882 |
0.849 |
|
Female |
5.670 ± 0.333 |
5.000 ± 0.577 |
5.670 ± 0.333 |
6.000 ± 0.577 |
4.330 ± 0.667 |
0.233 |
Table 2: Shows the changes in the differential leucocyte counts (as percentages) in response to PEN and Cu-NP exposure. Neutropaenia and lymphocytosis were characteristic features in both male and female Tilapia zillii in the PEN (I), PEN (II) and Cu-NP (II) groups. On the other hand, no significant changes were observed in the percentages of eosinophils, basophils and monocytes following PEN and Cu-NP exposure.
|
Sex |
Group/ Parameter |
Control |
PEN (I) |
Cu-NP (I) |
PEN (II) |
Cu-NP (II) |
P value |
|
Male |
MN (%) |
3.000 ± |
15.330 ± 0.882b |
4.500 ± 0.500c |
24.67 ± 2.028a |
5.000 ± 1.080c |
0 |
|
0.707c |
|
|
|
|
|
||
|
Female |
2.330 ± 0.333d |
15.330 ± 0.882b |
4.330 ± 0.333c |
24.670 ± 0.882a |
5.330 ± 0.333c |
0 |
|
|
Male |
Binucleated nucleus (%) |
0.250 ± 0.250b |
1.670 ± 0.667b |
0.500 ± 0.500b |
6.000 ± 0.577a |
1.500 ± 0.289b |
0 |
|
Female |
0.670 ± 0.333c |
8.670 ± 0.882b |
0.670 ± 0.333c |
15.330 ± 0.333a |
1.670 ± 0.333c |
0 |
|
|
Male |
Heart shaped nucleus (%) |
0.250 ± 0.250b |
1.330 ± 0.333b |
1.500 ± 0.500b |
6.000 ± 0.577a |
1.000 ± 0.408b |
0 |
|
Female |
0.670 ± 0.333c |
5.000 ± 0.577b |
1.670 ± 0.333c |
12.330 ± 0.882a |
1.670 ± 0.667c |
0 |
|
|
Male |
Kidney shaped nucleus (%) |
2.000 ± 0.707d |
8.330 ± 0.882b |
5.500 ± 0.500c |
20.670 ± 1.202a |
4.000 ± 0.707cd |
0 |
|
Female |
1.670 ± 0.667c |
8.330 ± 0.882b |
2.670 ± 0.333c |
13.330 ± 0.667a |
4.670 ± 0.333c |
0 |
|
|
Male |
Notched nucleus (%) |
1.750 ± 0.854b |
3.670 ± 0.333b |
2.500 ± 0.500b |
9.330 ± 0.882a |
2.250 ± 0.250b |
0 |
|
Female |
1.670 ± 0.333c |
3.670 ± 0.333b |
2.330 ± 0.333bc |
9.330 ± 0.882a |
2.000 ± 0.000c |
0 |
|
|
Male |
Irregular shaped nucleus (%) |
1.000 ± 0.000b |
2.670 ± 0.667b |
2.000 ± 0.000b |
11.330 ± 1.453a |
1.500 ± 0.500b |
0 |
|
Female |
1.000 ± 0.000d |
7.330 ± 0.882b |
2.670 ± 0.333d |
13.300 ± 0.667a |
4.670 ± 0.333c |
0 |
Table 3:Effects of penconazole and copper Nano formulated fungicide on the percentages of micronuclei and erythrocyte nuclear abnormalities.
Citation: Osman AGM, Elmileegy IMH, Farrag MMS, Said RE, et al. (2019) Haematotoxic and Genotoxic Potential of Penconazole and Copper Nanoparticles On Redbelly Tilapia (Tilapia Zillii). Open Acc J Agri Res: OAJAR-100019.