Emerging Infectious Diseases and Diagnosis Journal
Research Article
Distinctive Features of Microvesicles as A Transporter of GTP and Iron to Empower Pathogenesis of Mycobacterium Tuberculosis H37Rv
Shivangi, Kevlani N and Meena LS*
CSIR-Institute of Genomics and Integrative Biology, Mall Road, Delhi-110007
*Corresponding author: Laxman S. Meena, CSIR-Institute of Genomics and Integrative Biology, India, Tel: 011-27002200, Fax No: 011-27667471; E-mail ID: meena@igib.res.in
Citation: Shivangi, Kevlani N and Meena LS (2019) Distinctive features of Microvesicles as a transporter of GTP and iron to empower pathogenesis of Mycobacterium tuberculosis H37Rv. Emerg Infect Dis Diag J: EIDDJ-100009
Received date: 18 November 2019; Accepted date: 25 November 2019; Published date: 28 December 2019
Abstract
Mycobacterium tuberculosis (M. tuberculosis) maintains their own regulation machinery to allow its persistence for prolonge time till disease progresses. M. tuberculosis utilizes several means to adjust in recipient cell machinery for accommodating itself into the cell for its own survival that remains elusive in tuberculosis (TB) biology. Many intracellular bacterial pathogens naturally release microvesicles (MVs) under a variety of growth environments. These MVs are packed with immunological compounds, which help in modulating immune response. They show various biological biomarkers on their surface which may be helpful in recognizing them easily and may be helpful as a drug target. MVs have ability to cause disease in the presence or absence of live cells. Also, these MVs plays a major role in transportation of major signaling molecule known as GTP binding proteins which easily demonstrates its role as one of the important part of cell signaling and derive expressions of various genes. Thus, in the current study we are focusing on the possible strategies of MVs that may potentially involve in pathogenesis with an aim to be used in development of new therapeutic approach.
Keywords: Aveolar macrophages; GTP; Mycobacterium tuberculosis; Microvesicles; Pathogenesis; Phagolysosome
Abbreviation
ARAB : Arabinogalactan
BCG : Bacillus Calmette–Guérin
GTP : Guanosine tri phosphate
HIV : Human Immunodeficiency Virus
IFN-γ : Interferon gamma
IL-2 : Interleukin- 2
LAM : Lipoarabinomannan
M. tuberculosis : Mycobacterium tuberculosis
MVs : Membrane Vesicles
Ptp : Protein tyrosine phosphatases
SDG : Sustainable Development Goals
RRTB : Resistance to Rifampicin Tuberculosis
TCR : T- Cell Receptors
v-SNARE : Vesicle-soluble N-ethylmaleimide-sensitive factor attachment protein receptor
Introduction
Tuberculosis (TB) is a worldwide endurance disaster for declining percentage universally among demises caused by all other diseases [1]. About 33% of the aggregate populace is contaminated with Mycobacterium tuberculosis (M. tuberculosis) which is the causal representative of TB and shows highest mortality rate than any other bacterial pathogen. The death rate of TB classifies this disease as one of the top 10 deadly disease worldwide. In year 2016, it was predicted that 1.3 million TB patients were pass away among Human Immuno Deficiency Virus (HIV) co infected individuals, but in year 2000 this data was around 1.7 million. In the same year, there were 6 million (including previously noted and novel cases) had been found to get resistance for rifampicin (RRTB), which is the most efficient first-line drug for TB, out of which 4 million been developed multidrug-resistant TB (MDR-TB). Approximately half (47%) of these cases were only classified in India, and countries like China and Russia which also shows drastic number because of their population [2]. The first objective of the “stop TB plan” is set for 2020 which include principles like SDG targets involves to half the number of deaths and injuries from traffic accidents, decrease the death rate of communicable diseases like tuberculosis and decrease the harm caused by alcohol. In 2017, there was 35% decline in TB deaths and a 20% fall in TB prevalence, compared with intensities in 2015. In 2017, WHO has also developed a TB- Sustainable Development Goals (SDG) monitoring agenda of 14 indicators that are associated with TB incidence, under seven SDGs [3]. As early as in 1994, there was a report that portrayed capacity of Mycobacterial species (Mycobacterium avium/Mycobacterium tuberculosis) to transport bacterial components, particularly those molecules that favors the virulence character of this pathogenic bacterium for example membrane components like lipoarabinomannan (LAM), arabinogalactan (Arab) in particular intracellular vacuoles rather than the bacteria enclosed compartment [4]. The majority of the bacteria including M. tuberculosis releases small membrane bound vesicles that may contain genetic material, proteins lipids etc. which help these vesicles to interact easily with surrounding environment and to take positive response towards spreading infection. The production of microvesicles and communication mediated by them is thought to be conserved in unicellular and multicellular organisms. The exceptional achievement of M. tuberculosis like pathogen is closely linked with their capability to prevail in recipient cells in latent form for extended periods without causing death of host cell [5]. In addition to this, the recent appearance of highly drug-resistant strains possess an even greater threat like multi-drug resistance (MDR), totally drug resistant (TDR) and extremely-drug resistance (XDR) [6]. Dendritic cells uptake M. tuberculosis and move to lymph node from lungs where it starts colonizing and a granuloma like structure is formed which then travelled towards lymph nodes [7]. Many intracellular bacterial pathogens obviously discharged microvesicles (MVs) in response to some signal or may be in stress conditions. Bacterial MVs formerly accounted in the 1960s in Escherichia coli bacteria [8,9]. MVs are the small membrane enclosed sacs which are thought to be shed from the cell membrane and outer membrane of various types of cells [10]. It has been shown that MVs can alter signaling of the recipient host cells either by immunogenic or a covalent interaction of these vesicles with immune cells or uptake by recipient cells. These vesicles had been appearing to control the synthesis of nearby recipient cells in assorted manner, by controlling inter cellular signaling pathway for presenting novel assets ensuing to the fulfillment of new receptors, chemicals and still hereditary substance from the vesicles [11]. In one of the study, MVs also associate with M. tuberculosis pathogenesis. Although vesicle production was also seen in nonpathogenic and fast growing bacteria therefore it is concluded that vesicle production is the conserved phenomenon in Mycobacterium species. In this review we focus our attention towards the production of MVs released by gram positive and gram negative bacteria and their importance in generating an active immune response due to which they might be serve as potential drug target.
Biology of MVs
For pathogenic bacteria there are strong evidences that released MVs represents one of the major delivery mechanisms for the release of immunologically active molecules that contribute to virulence. MVs are the intricate composition poised by a lipid double layer system which outlines circular structure that contains lumen assortment in range from few nm to 500 nm in diameter [12]. These MVs are produced by eukaryotes, archaea and bacteria which confirm that they are universal structures [13]. In addition of comprising of lipid molecules these vesicles also include proteins that act on transmembrane region or proteins that encircle soluble hydrophilic components which are the component of the cytosol of the contributor cell [14]. For gram negative bacteria, it was observed that these vesicles shed off from the external membrane of the bacteria over and above therefore they are called as outer membrane vesicles (OMVs) [15-17]. In gram positive bacteria, various terms are used in place of MVs like ectosomes, shedding vesicles, extracellular vesicles etc. In case of M. tuberculosis, there is a covalent linkage between peptidoglycan and arabinogalactan, which then intermingled with mycolic acids. The superior segments of the outermost layer of this bacterium consist of free lipids which in turn are covered by outermost layer of capsule comprising polysaccharides, proteins and lipids that play role in important MVs formation [18]. M. tuberculosis also produces MVs in the culture for example in lung alveolar macrophages cell lines. Some MVs were also found to carry DNase-resistant intra vesicular DNA, confined through a phospholipid bilayer membrane and strongly contribute in the virulent character of bacteria. Studies revealed that, MVs have been exposed to carry definite mRNAs, different small RNAs [19-21], DNAs [22-26], toxins, communicative compounds and also nutrient scavenging factors from one cell to another cell [27-29]. These proofs proposed that M. tuberculosis extracellular vesicle is might be an important way to convey immunologically dynamic M. tuberculosis harmful elements. By the side of medical relevance, these vesicles can be used as a material in immunization improvement to defend TB since M. tuberculosis MVs might be used to inspire insusceptible reactions with occlusion of adjuvants [30]. In expansion, vesicle-related antigens of this bacterium are measured as probable affectionate biomarkers [31].
Immunological aspect of host against MVs
In previous studies, it was found that these MVs may also pack with immunologically active compounds which help in modulating immune response and help in transferring immunologically active molecules from an immune cell to a non-immune cell. They are also said to be as active immune mediators. These vesicles are fully capable of causing disease in the presence or absence of living cells. In one of the study it has been shown that, immunization with MVs from Bacillus Calmette-Guérin (BCG) and M. tuberculosis extract, an assorted humoral and cellular immune response heading for mutually membranous and cell wall apparatus, like lipoproteins. On the other hand, merely immunization by M. tuberculosis MVs are capable of protecting host as the live BCG immunization [32]. The proteomic investigation revealed that in M. tuberculosis, there are almost 50 proteins which are augmented through proteins involved in virulence together with Toll like Receptor 2 (TLR2) ligands such as Lipoproteins (LpqH, Lpr A and LprG). MVs help M. tuberculosis by providing its entry into the macrophages after recognizing the exact pathogen associated molecular patterns (PAMPs) on phagocytosed pathogen and also release pro-inflammatory cytokines like tumor necrosis factor alpha (TNF-alpha) and interleukin 10 (IL-10) that generate neutrophils, immature monocytes and dendritic cells in lungs. Mycobacterial Lipoarabinomanon (LAM) in addition to phosphatidylinositol mannoside (PIM) initiates and gathers within LAMP-1 constructive vacuoles in late endosomal compartments and multivesicular bodies (MVB), which soon after fuse with the cell layer to discharge LAM/PIM-containing MVs in the extracellular conditions [33]. The discharged vesicles can exchange bacterial segments within adjacent non infected macrophages. In one of the study of Schorey used stream cytometry to uncover a positive relationship of discharged vesicles along with endosomal indicators, Lysosomal-associated membrane protein 1 (LAMP1), Lysosomal-associated membrane protein 2 (LAMP2) and Major Histocompatibility Complex II (MHC-II), like the exosomal-marker, tetraspanin, Cluster of Differentiation 81 (CD81) [34]. In his study, he embroiled a system in which the intracellular growing bacteria could convey their particular signal with other host cells. MVs accused of mycobacterial constituents can animate naive macrophages in a pro-inflammatory way, by provocative the conception of TNF-α, synchronized by activation, usual T cell articulation, buried (RANTES) and also inducible nitric oxide synthase (iNOS) [35]. Also, these vesicles can stimulate CD4+ and CD8+ T cells equally, reliable through development of solid insusceptible reaction and demonstrate an option course of antigen introduction in these recipient cells in lieu of MHC introduction through macrophages as well as dendritic cells [36]. At this point, here emerge several methods within which MVs might interact with additional cells. Especially, vesicles drop as M. tuberculosis tainted APCs demonstrate MHC-II complex as well as show capability of presenting processed extracellular antigens along with CD4+ cells, via T cell line clonally constrained to M. tuberculosis Antigen 85B [37]. In later studies, it had been revealed that vesicles in addition restrain complete M. tuberculosis proteins, in addition Antigen 85 complex proteins, heat shock protein X (HspX), chaperone protein DnaK, and an amount of additional M. tuberculosis proteins [38]. Vesicles discharged from M. tuberculosis tainted macrophages additionally show inhibitory effect on cell resistant reactions related with defensive invulnerability, particularly impeding interferon IFN-γ synchronized channel that initiate naive macrophages [39]. The endurance of M. tuberculosis inside recipient cell is a mere equilibrium of protected establishment system for example mandatory for phagocytosis throughout precise receptors and escaping the intrusion of union of phagosome lysosome [40]. From the above study, it is obvious that MVs might play more important role than was originally assumed.
MVs at the genome level
It had been also found that MVs production through M. tuberculosis is under genetic control [41]. In a current revised data, virR gene of M. tuberculosis is documented like manager of unaffected inflection and vesiculogenesis within M. tuberculosis [42]. Interruption of this gene enhances immunity providing substances fabricated by mice and human macrophages in reaction of a bacterium which came out as a satisfied phenotype in macrophages and mice. On the other hand, virR deleted mutants comprises of nil development deficiency in liquid background, which propose function of virR gene in virulence of M. tuberculosis. It was seen that virR gene manage the liberation of immunomodulatory compounds, like lipoprotein LpqH, by way of MVs since there was not a single authentication that deficiency of virR gene globally augment secretory pathways that are not mediated by MVs but mediated by SecA2 and Tat. These MVs cooperate as an imperative modulator in host pathogen interaction and mediate release of various secretory molecules to the surrounding environment. It had also proved that these MVs is novel form of transformation in eukaryotes as they transform the inherited symphony of beneficiary group and modify roles of recipient cells [43-45].
Interrelation between iron and MVs
One of the factor require for release of these MVs is limitation of iron in the host. Iron is a vital supplement for all organisms with just a couple of exemption like, Vibrio cholerae, and Brucella melitensis [46]. These all marked as maximal manufacture of vesicles in late log phase, which is the phase matched with lower availability of iron in the medium. Iron is an essential molecule for various catalytic activities associated with metabolic mechanism performed by our cellular system. The competence to get iron inside the cell is a fundamental property of all living organisms although this phenomenon introduces a dispute since iron molecule is present as an insoluble form in the aerated condition and at unbiased ionic strength. Thusly, iron is not present in the secreted form and therefore also found as a hurdle to deliver proteins in the iron deficient cellular proteins. For answering these disputes, disease causing organisms have developed various approaches to facilitate and permit these organisms for contend iron inside the cell and to ascertain the conquering infectivity. On the other hand, since frequently in some matter, higher amount of any required item could be terrible. Multipotent iron acquirement should be compactly prohibited since overload of liberated iron molecules create a severe hazard for the living organisms owing to its involvement in a chemical effect that can cause production of lethal variant of oxygen molecule by common harvests of aerobic mechanisms occurring inside the cell (Figure 1) [47].
During the occurrence of oxygen and at unbiased pH, iron is less soluble in water and therefore ferric iron atom is not set up as free of charge instead this atom is requisition in complex form through recipient iron binding proteins, such as transferrin, lactoferrin, and ferritin [48]. Since iron is indispensable for cell survival, elevated resemblance of iron attainment systems is important and intended for antigens to propagate throughout contagion. In an unbound condition, quick extraction of available ferrous atom is the main exceptional occurrence of nutritional defense and on the additional side, insufficiency of iron in the recipient is an indication of pathogens to encourage emergence of extermination and their extra virulence factors to come in effect subsequently with iron attainment systems [49]. Similar to several microbes, M. tuberculosis produces siderophores to confine iron. Siderophores show large binding capacity for iron and work as iron chelators so as to combine with ferric iron atom. Siderophores belonging to M. tuberculosis are salicylate containing compounds which named as mycobactins essential for virulence [50]. M. tuberculosis secreted two types of mycobactins, one is carboxymycobactin which is the released form and second one is mycobactin which is present as cell linked state (Figure 2) [51].
Carboxymycobactin shows the capability to eliminate mcobactin atom which remain as crypt with the host iron requisite protein [52]. Avirulent Mycobacterium species such as M. smegmatis generate exochelin as their iron chelators and little amount of carboxymycobactin instead of producing carboxymycobactin only in large amount [53,54]. Carboxymycobactin are the extracellular counterparts of mycobactins that possess the main structure as mycobactins but also contains a carboxyl chain carboxyl side chain which makes its character as secreted and thus makes more potent iron transport for excising virulent character of the bacterium. The critical character of siderophores intended for iron attainment is confirmed through the production of distorted strain (mbtB) of M. tuberculosis that is incapable to generate either of the carboxyl mycobactin or mycobactin. This mutation weakens the capability of M. tuberculosis replication in media containing low amount of iron in tainted macrophages of the cell [55]. These MVs are helpful in transferring of mycobactins from one cell surface to other cell surface and therefore fulfilling the requirement of iron of M. tuberculosis.
Assumption related to microvesicles
There are three probable mechanisms that explain non-mutually special devices through which MVs pass through solid cell walls and these hypotheses are as follows:
The copious virulent factors including early endosomal Antigen-1 (EEA1) a tethering molecule plays a crucial responsibility during phagosome maturation via interrelating straightforwardly through syntaxin-6 protein sphere which is soluble N-ethylmaleimide-sensitive aspect affection protein receptor (SNARE) establish in endosomal transport vesicle comprises v-SNARE that concerned in the liberation of MVs [59]. Synaptosomal-associated protein (SNAPs) are the proteins which are reported for intended explicitly of membrane combination to directly accomplish union through creating a taut comprehensive through t- SNARE.
Role of GTP binding proteins in transportation of these microvesicles
GTP-binding proteins are small 20 to 30 kDa molecular mass protein that functions as molecular switches in cellular signaling events. The role of these proteins in pathogenesis is believed to regulate various actions like formation, transportation of virulent factors along with union of MVs by the plasma membrane. The MVs which contain toxins, adhesion molecules and fractions of M. tuberculosis, proceed as a different mode for transporting these dynamic after maturing off by early phagosome to facilitate the pathological events. Once formed, the MVs moves to the destination membrane and fuse with it. The use of GTP/GDP cycle events in MVs tethering had controlled by Rab/Ras GTPase activating proteins (Figure 3).
The Phagocyte contains multiple membrane associated adhesion molecules receptor i.e. integrin (α5-β1) involve in attachments of bacilli that, followed phagocytosis mechanism. The early phagosome containing M. tuberculosis employs some soluble proteins kinases (PknG and PknF are serine/threonine protein kinases) to cause inhibition of phagosome-lysosome fusion. For instance, phagosome contained M. tuberculosis secret some active PtpA intracellular, which change the acidification within phagosome to inhibit the V-ATPase activity (control H+ proton pump) using energy from ATP-hydrolysis. In addition to this, other kinases (PknG and PknF) with abrupt expression and retention of Rab, and Tryptophan-Aspartate containing coat proteins (TACO)/ coronin-1 proteins involve in to inhibit phagosome-lysosome fusion through a mechanism that is yet to be defined but possibly involves in mycobacterium survival. Translocation of MVs is an alternative way to transport these factors via v-SNARE to t-SNARE complex formation resulting in budding off of MVs by using the GTP/GDP cycle events in MVs tethering, which controlled by Rab/Ras GTPase activating proteins. Once MVs budding off within macrophage cytosol, the MHC-II processed antigen presentation event before their presentation that interact with the TCR on CD4+ T-cells to drive antigen-specific T-cell responses i.e. secretion of IFN-γ and IL-2 which leads to macrophage activation and T-cell proliferation. Moreover, the processed MVs peptides then traffic from phagosome vacuoles to exosomes that, in turn, are released from the compartment of host cells.
Diverse Rab GTPase proteins are contained in the cytosolic countenance of a precise intracellular membrane, wherever these proteins play like controllers of discrete points in membranous pathways [60]. Protein present in the intracellular compartments and lipid passage is an elemental practice mandatory for the invention of dedicated membrane organized organelles like MVs and the communications among them. Vesicular transport is single variety of announcement involving organelles which involve in union of membrane with the conveyed vesicle in addition to the intentional targeting membrane to establish the accurate interaction [61]. It has been demonstrated that Rab proteins are molecular controllers remain in a dynamic condition in their GTP fixed state and as dormant in their GDP fixed conditions [62-65]. During the active condition, Rab proteins recruit a diverse group of proteins termed effectors. Enrollment of effectors protein might be enabling Rab proteins for directing the major steps in vesicular transportation as well as cargo assortment, promising, progress, interaction and union [66]. The Rab protein does not have large inherent activities as GEF or GAP shows. These proteins are synchronized through additional guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs), in their GDP form and these proteins are classically soluble and in addition they remain bind with guanine nucleotide dissociation inhibitor (GDI) [67].
The current review highlights the concept of MVs arisen within early phagosome containing M. tuberculosis specific receptor required for activation and docking mechanism. The highlights about these MVs also find its crucial features which prove this to be a potent targeting molecule in response with the drug development therapies.
Conclusion
It has been shown that in gram positive bacteria like M. tuberculosis as well as in other bacteria, proteins buried by way of various pathways are essential for communication between cell, play crucial role to come in contact, nutrient attainment, non toxification of the outer environment furthermore virulence [68,69]. Conversely, this is uncertain that MV’s consequence is restricted to tainted macrophage or something else might be as well responsible on behalf of M. tuberculosis intracellular endurance. The formation of MVs is an essential part derived under varying conditions or is purely the outcome of perfunctory recipient decease or the unconstrained by an apoptotic cell in infection by M. tuberculosis. However, it was thought that these vesicles found in culture supernatants of the infected cell with M. tuberculosis [70,71]. Furthermore, these are widely considered as one of the crucial feature in both the complex organisms as well as for the prokaryotic cell biology because of their capacity to alter the functionality and contribute into pathology following infection, which remained unrevealed. The involvement of MVs in different pathological aspects of M. tuberculosis pathogenesis emerge as a relatively wide spread process in TB. The increase interest in MVs-based intercellular communication, it has been implicated that these are potential secretory vesicles containing toxins; adhesion molecules etc. and operate as a substitution approach for transporting these aspects by maturing from early phagosome containing M. tuberculosis to facilitate the pathologic events (Figure 1). Moreover, phagosome harboring M. tuberculosis possesses numerous strategic molecules and kinases secretion which inhibit phagosome maturation and bactericidal action. Even though the reading of MVs creation in various microorganisms including M. tuberculosis has exaggerated, the whole machinery of its generation through the cell wall remains inadequately unspoken, and how controlled mechanism of their release occurs within the cell is still remain an essential question. Over the above, MVs distribution throughout macrophage infection, GTPase activities in response to GTP-binding proteins are also an important resource for our understanding in the pathogenicity aspects of bacilli at different infection’s level. In conclusion, the packing mechanism of MVs followed infection is poorly understood and involvement of these immune powerful energetic particles that could amend immune reaction for the advantage of the M. tuberculosis survival [72].
Future studies
Intracellular pathogens like M. tuberculosis with the intention of entering inside the recipient cell during the phagocytic mechanism comprises deployed mechanism for contradicting the acidic surrounding within phagosome. These MVs help this bacterium to retain in host phagosome and provide all nutritional supplements essential for its replication. So, blocking MVs generation at the genetic level, hinder MVs interface with their objective recipient cell and also molecular transfers which might be the potential source of information that can use as therapeutic approach to target pathogenesis of M. tuberculosis. Perhaps the interesting topic of research, the mechanistic insights behind the function and biogenesis are still remained as a question. So hopefully this review would become successful in scattering the knowledge and importance of these vesicles in therapeutics and drug development industry.
Acknowledgement
The authors acknowledge economic sustain from the project GAP0145 of the Department of Science and Technology (SERB). We also acknowledge the support from scientific and administrative staff of CSIR-IGIB.

Figure 1: Fenton Reaction: In the presence of hydrogen peroxide which is metabolic product of aerobic respiration, the excess of the ferrous iron present in the cell converts into ferric iron and also generate toxic free radicals (Fenton reaction).
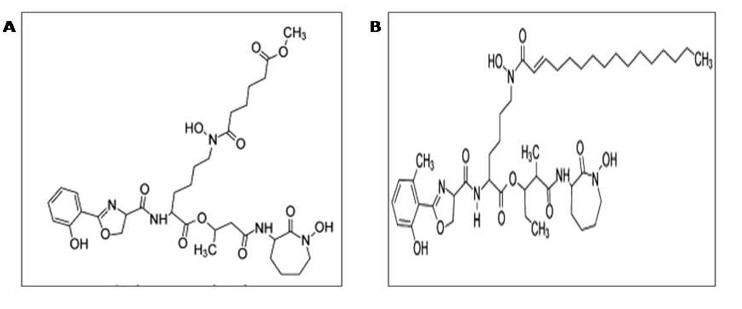
Figure 2: Siderophores of M. tuberculosis (Mycobactin and carboxymycobactin): Mycobactin is an intracellular lipophilic siderophore whereas carboxymycobactin is an extracellular siderophore. Carboxymycobactin are the modified group of mycobactins bearing a short alkyl side chain of variable length and unsaturation. They incorporate a terminal methyl ester motif, which enhances polarity and solubility and is essential for iron chelation. Both mycobactins and carboxymycobactin are salicylate derivatives with one modified Ser/Thr and two Lys molecules added.
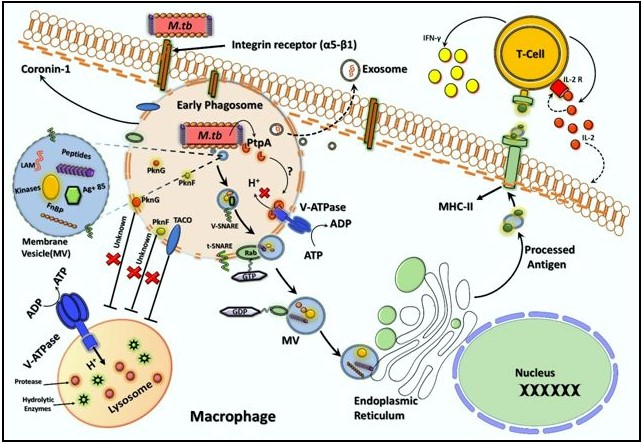
Figure 3: Role of cellular compartments in phagocytosis and intracellular effects of Microvesicles (MVs) within the phagocyte macrophage cell in response to M. tuberculosis.
Citation: Shivangi, Kevlani N and Meena LS (2019) Distinctive features of Microvesicles as a transporter of GTP and iron to empower pathogenesis of Mycobacterium tuberculosis H37Rv. Emerg Infect Dis Diag J: EIDDJ-100009