Annals of Medical & Surgical Case Reports
Case report
Accidental Overdose of Paliperidone Long Acting Injection Formulation (LAI): Symptoms, Cause and Pharmacokinetics
Koch HJ *
Department of Psychiatry and Psychotherapy, Heinrich Braun Hospital Zwickau, Germany
*Corresponding author: Horst Josef Koch, Department of Psychiatry and Psychotherapy, Heinrich Braun Hospital Zwickau, Germany, Tel: +49 375 510; Email: horst.koch@hbk-zwickau.de
Citation: Koch HJ (2019) Accidental overdose of paliperidone long acting injection formulation (LAI): symptoms, cause and pharmacokinetics. Ann Med & Surg Case Rep: AMSCR-1000029
Received date: 08 November, 2019; Accepted date: 15 November, 2019; Published date: 23 November, 2019
Abstract
Xeplion R is a long-acting injectable (LAI) antipschotic (paliperidone palmitate) which generally warrants therapeutic plasma levels over 4 weeks. Overdoses or intoxication, especially with medical sequelae, are very rare. A 60-year old schizophrenic patient developed a confusional state with anticholinergic components after anew injection of 100 mg after self-willed interruption of injections with maximum plasma levels of about 170 ug/ml. The estimated interval half-life was 7.5 days based on a 1st order kinetic model. He improved with decreasing plasma levels and administration of lorazepam. 75 mg LAI paliperidone were well tolerated and effective later on.
Keywords: 1st order kinetics; Confusional state; Half-life; Overdos; Paliperidone palmitate (LAI)
Introduction
The metabolite of risperidone paliperidone (9-OH-risperidone) is meanwhile primarily administered as a long acting injection formulation (LAI) in Germany (XeplionR, Fachinformation 2018). Due to its OH-group it is suited for esterification – palmitate - and hydrolysis in the tissue after intramuscular injection. Tmax is achieved within roughly 13 days after injection. Deltoid injections yield some 28% higher concentrations compared to gluteal administration. Slim persons show slightly (10 - 20 %) higher plasma levels. The mean apparent half-life of the depot is between 25 and 49 days (Summary of Product characteristics, Annex 1). It is a D2- and 5-HT2A- antagonist with antagonist activity in α1, σ2 or H1 receptors, too [1]. It is said to be free of anticholinergic properties.
Reports of intoxications or overdoses with paliperidone LAI are rare and except one case refer to the formulation with accidental overdose [2]. A 22-year-old man African American who received a total dose of 624 mg im without significant side-effects after 14 days monitoring. However, combination of olanzapin long-acting injection with an inadvertent oral dose of 18 mg paliperidone instead of 9 mg provoked anticholinergic symptoms in a 19-year-old female with a history of cannabis consumption including confusion, religious delusion and tachycardia [3]. Massive overdoses with oral paliperidone provoked toxic syndromes which may at least to some extent correspond to anticholinergic activity such as tachycardia, hallucination or confusion [4].
Case report
A 60-year-old patient (170 cm, 66 kg) was referred to ward due to an exacerbation of his psychosis with paranoid thoughts and auditory hallucinations. He had been treated with 100 mg paliperidone long acting injection formulation (XeplionR) for many years and went well with regard to his psychosis but refused injections (100 mg every 4 weeks) two months before admission. After admission he agreed to the injection of 100 mg paliperidone LAI [5]. However, after approximately one week his psychopathology-after an initial amelioration – deteriorated again. He now developed a delirious syndrome with confusion, sweating, optical hallucinations, fluctuations, impaired day-night rhythm, slight mydriasis and tachycardia. No medical problems, particularly no renal or liver impairment, occurred. He initially got a single dose of haloperidol without effect but starting administration with lorazepam up to 1 mg four times a day improved his condition within a few days. We controlled plasma levels and after normalization of the plasma levels LAI treatment was continued with 75 mg every 4 weeks, which was well tolerated and clinically effective. The patient gave his informed consent for additional blood samples and publication. He was discharged to the out-patient clinic after two months and remained in reasonably good health for more than 6 months [6].
We determined blood levels of paliperidone which showed increased levels up to 170 ug/ml, probably somewhat higher before blood sampling. Therefore, we stopped XeplionR injections of 100 mg and observed the course of concentration during the next weeks. The course of the paliperidone levels after admission is given in Figure 1.
Pharmacokinetic model
A linear regression model [C=kt+Ao] yielded the best fit of the concentration (c) versus time (t) data. Ao denotes the initial concentration at to = 0 and k the (negative) slope (rate constant). In order to get the half-life T1/2 of the zero-order kinetic model we calculate Ao/2=Ao-kt which leads to T1/2 = Ao/2k. All calculations were done with commercially available software (NCSS Statistical Software, Version 07.1.21, NCSS LLC, Kaysville, USA). Figure 1 shows the result of the regression which yielded a well fitted curve (r=0.85) which allowed to estimate the half-life as follows: T1/2 = 149 / (2*4.5) ~ 7.5 days [7].
We continuously monitored the patient (ECG, clinically, vital signs) until the patient had clinically completely recovered and the concentration approached the therapeutic window (5-100 ug/). As he has had a benefit from the medication, we agreed to treat the patient with a lower dose (75 mg every four weeks) and planned regular laboratory controls including blood levels [8-10].
Discussion and Conclusion
Intentional or accidental overdose with paliperidone long-acting injection formulation is a very rare complication. The management with lorazepam and monitoring proved to be sufficiently safe. Treatment was continued with a lower dose which was well tolerated and efficient as well plasma levels being within the therapeutic window. A zero-order kinetic model yielded a plasma half-life of about 7 to 8 days which is lower than that found in long-term kinetic studies. However, it might explain the probably the rapid increase of plasma levels due to the shorter half-life. The reason remains speculative but comparable low weight or injection site, motion of the limb or local blood flow may be influencing factors. In such cases out-patient monitoring may be suitable to find the correct dose and possibly injection interval.
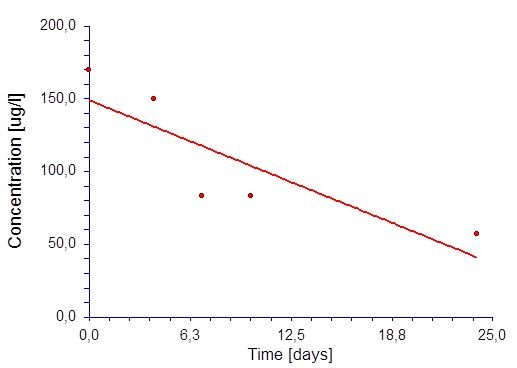
Figure 1: Concentration versus time plot with following model (y=mx+b) characteristics: R=0.85; estimation of characteristics: Co = 148,9 ug/l; m=-4.49.
Citation: Koch HJ (2019) Accidental overdose of paliperidone long acting injection formulation (LAI): symptoms, cause and pharmacokinetics. Ann Med & Surg Case Rep: AMSCR-1000029.