Journal of Sports Science and Physical Therapy
Review Article
Review on Phage Therapy as Alternative Treatment for Meticillin-Resistant S. Aureus (MRSA) Infections: Identification and Application of Phage
Feyissa N1, Jirata D2, Alemu T2 and Desalegn A2
1Department of Veterinary Laboratory Technology, College of Agriculture and Veterinary science, Ambo University, Ethiopia
2Addis Ababa University, College of Natural and Computational Science, Department of Biology
*Corresponding author: Negassa Feyissa, Department of Veterinary Laboratory Technology, College of Agriculture and Veterinary science, Ambo University, Ethiopia, Tel: +251933649741; E-mail: negassa.feyissa@yahoo.com
Citation: Feyissa N, Jirata D, Alemu T, Desalegn A (2019) Review on Phage Therapy as Alternative Treatment for Meticillin-Resistant S. Aureus (MRSA) Infections: Identification and Application of Phage. J Sports Sci Physical Ther: JSSPT-104.
Received date: 06 August, 2019; Accepted date: 15 October, 2019; Published date: 25 October, 2019
Abstracts
The emergency of drug resistant bacteria is becoming the burning issue of the globe in public and veterinary medicine. This is because of the reasons of that the rate at which the bacteria develop resistant to antibiotics exceeds the rate at which new drugs are developed; and the multidrug resistance development of some bacteria. Different international organizations have, therefore, urged the scientific communities including research institutions to design novel strategies to combat the problem before the end of the era of antibiotics. Methicilin resistant Staphylococcus aureous (MRSA) is among such bacteria affecting human and animal and swiftly developing resistance to all β-lactam antibiotics and recently determined resist to vancomycin (the most effective antibiotic of choice against the pathogen). If, therefore, such situations are left aside without finding alternative treatments, the nature of MRSA will empower it to be serious public importance in the world. It is obvious that MRSA is residing on the body of human and animals as microflora on skin, in mouth, upper respiratory tracts and urogenital tracts. These phenomena led the scientific community to look for new strategy other than chemical drugs to fight against MRSA. To do so they attempted to look back into the past to bring solution in the future. They found bacteriophage the preferable method to chemical drugs for different reasons. Despite there are obstacles and doubts of phage therapy for MRSA infections, biotechnological strategies have been given great trust to solve the problem to bring the best solution for the current obstacles in phage therapy.
Keywords: Infection; MRSA; Phage therapy
Introduction
The emergence of many important drug resistant bacteria has been becoming bottle neck for the current pharmaceutical sciences and antimicrobial resistance (AMR) is currently being the global issue. The problems associated with AMR have been more exacerbated with different factors. Primarily, many of bacteria which developed resistant to a specific antibiotic are prone to develop resistance to related drugs of choices (multi drug resistant). Secondly, the rate the pathogen is developing resistance to antibiotics is faster than the rate the scientists are developing new alternative drugs and/strategies. Particularly, the former factor has led the world to despair to rely on chemical drugs to combat the pathogen [1]. As the result WHO (2017) has urged that unless new drugs are developed or new strategies are designed, our enemy (the pathogenic bacteria) will re-emerge to cause worldwide diseases.
One of the most important of such bacteria which has been approximating to the end of lacking effective drug is a Gram positive cocci developing resistant to the most effective drugs of choice are methicillin-resistant Staphylococcus aureus (MRSA) strains of S. aureus. Methicillin is among the most effective antibiotics against strains of S. aureus which have developed resistant to other antibiotics including β-lactams such as penicillin G, ceftiofur sodium, cloxacillin, cephapirin, and ampicillin [2]. The bacterium is the most important pathogen associated with simple diseases such as pimples and boils to serious infections such as wound infections, pneumonia, or septicaemia (infections getting into the bloodstream) which can result in life-threatening illness [3].
The most important feature of MRSA is that it resists to all beta-lactam antibiotics such as methicillin, penicillin G, ceftiofur sodium, cloxacillin, cephapirin, and ampicillin [3] and even less susceptible to vancomycin which is the only most effective antibiotic against the pathogen so far. These features of the bacterium have attracted the attention of the science community to design methods/strategies other than chemical drugs to combat the pathogen of which bacterial virus named as bacteriophage or phage has been frequently mentioned. The aim of the manuscript is, therefore, to review phage therapy as alternative treatment against MRSA infections. The review also discusses the application of phage and technologies used to improve phage therapy.
Antibiotic Resistance in Bacteria
In the last 2-3 decades’ drug resistance bacteria have been emerged with speed exceeding the development of new antibiotics. Now days, these bacteria have therefore, been becoming the bottle neck for clinician in the globe. AMR is so important that WHO, the European Parliament, the US Food and Drug Administration, and many scientific organizations of the world have acknowledged the elimination of drug resistance of microbes as a priority action. More importantly, some of these pathogenic bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) are multidrug resistant which leads to lacks the drug of choices for infection treatment. However,
discovery of new class of antibiotics have been steadily declined in the globe due to different reasons. The current analyses show that the search for new anti-infection agents conducted by pharmaceutical companies is becoming more restricted due to the growing costs of conducting the appropriate trials and low profits. In addition, the risk of investment to develop new antibiotics increases because of the fact that the pathogens can rapidly acquire resistance to the newly formulated chemical drugs. As the antibiotic pipeline threatens to run dry, the role of academic centers and scientific institutions engaged in developing new anti-infection technologies is becoming increasingly crucial. Therefore, new strategies other than chemical antibiotics need to be designed to combat the drug resistant bacteria.
Among the methods alternative to antibiotics and chemotherapeutics for combating bacterial infections, therapy using bacteriophages is frequently mentioned. Therefore, bacteriophage may be used as therapeutic agent to treat infections caused by multi-drug resistance bacteria. Even it can also be a promising alternative for alleviate the risk of transmitting pathogenic bacteria via food commodities.
Characteristics of Methicillin-Resistant Staphylococcus aureus (MRSA)
The most important species in the genus Staphylococcus is S. aureus. It is a Gram-positive coccus (1 μm in diameter) appearing microscopically as grape-like clusters due to incomplete three planar divisions, non-motile and produces golden yellow colonies from which the name was coined (aureus means golden). In the laboratory, it is characterized as catalase positive to catalyse hydrogen peroxide into water and oxygen and coagulase positive that causes coagulation of rabbit plasma relatively quickly. Moreover, it is facultative anaerobic, and grows abundantly under aerobic conditions. Under aerobic conditions, it produces acetoin as the end product of glucose metabolism and ferments mannitol. It produces thermonuclease, and is sensitive to lysostaphin. S. aureus is salt-tolerant (10-15%) and relatively resistant to drying and heat. The cell wall of S. aureus contains three main components: the peptidoglycan comprising repeating units of N-acetyl glucosamine β-1,4 linked to N-acetyl muramic acid; a ribitol teichoic acid bound via N-acetyl mannosaminyl-β-1,4-N-acetyl glucosamine to a muramyl-6-phosphate; and Protein A, which is covalently linked to the peptidoglycan and particularly is characterized by its ability to bind to Fc component of the immunoglobulin in plasma causing auto agglutination. Most of the other species of staphylococci lack protein A in their cell wall.
MRSA as Public and Animal Health Burdon
S. aureus is a common species of bacteria living on the skin and mucous membranes of humans and other animals as a commensal. However, sometimes it may cause infectious diseases ranging from mild skin infection to life-threatening sepsis in both animals and humans. The pathogenic strains of the bacterium are known in causing skin and soft tissue infections, wound infections, endocarditis, osteomyelitis, bacteraemia and chronic infections by forming biofilms in association with medical devices. Occasionally, the skin infections can spread to other organs of the body with more severe symptoms, including necrotizing fasciitis, and necrotizing pneumonia (tissue destruction), followed by sepsis and toxic shock, and then death in up to 50% of the cases. Staphylococcal food poisoning caused by S. aureus is also one of the most common types of foodborne illness worldwide and is mostly characterized by severe vomiting and diarrhea. The symptoms result from the fact that S. aureus produces a large number of toxins and enzymes, of which the heat stable enterotoxins are most important in the production of gastroenteritis.
In animals the S. aureu is an important cause of clinical and subclinical mastitis in dairy animals, otitis externa in pets, and joint infection leading to edema and arthritis in cattle. In most of the cases outbreak of S. aureu infections were reported in large stables and post-operative complications.
Besides to its pathogenicity, S. aureus is becoming important in the fact that it resists to antibiotics commonly beta-lactams such as penicillin, nafcillin, oxacillin, methicillin, and even vancomycin which categorizes it to one of the most non-treatable microbial in the globe. Particularly the emerging of methcillin resistant Staphylococcus aureus (MRSA) increases its intensity to be one of the global issues of health concerns due to the fact that methcillin is a semi-synthetic penicillins and the most effective antibiotic against S. aureus. It is a well- known pathogen occurring in human and veterinary medicine.
Most of MRSA strains are considered as resistant to all beta-lactam antibiotics such as penicillin G, ceftiofur sodium, cloxacillin, cephapirin, and ampicillin. Researchers have found that an increasing number of MRSA strains have developed reduced susceptibility to even vancomycin and are termed “vancomycin intermediate Staphylococcus aureus” (VISA) strains. It is obvious that vancomycin is the drug of choice for MRSA infections. Therefore, if MRSA strains are resistant to vancomycin, the infections caused by such strains may be fatal because of lack of alternative antibiotics. As the consequence, in the near future, there will be a lack of antibiotics available to treat MRSA-associated infections; or it may take long time to develop new drug against the pathogens.
Since strains of MRSA have spread between the groups, different types of MRSA strains may be distinguished based on epidemiological groups. As the result MRSA can be categorized as hospital associated MRSA (HA-MRSA), Health Care Associated Community MRSA (HCAMRSA), Community Associated MRSA (CA-MRSA) and livestock Associated MRSA (LA-MRSA).
HA-MRSA, also known as nosocomial pathogens, are types of MRSA whose infections caused by them are acquired in health care settings when the patients with particular risk factors such as prolonged in hospital stay, care in intensive care units (ICUs), prolonged antibiotic treatment, surgical interventions and/or close contact with MRSA-positive individuals. HCA-MRSA are strains of MRSA associated with outpatients colonized/infected by MRSA, and previous hospitalization, such as residence in a nursing home, receiving of home nursing, attending centers for dialysis and/or centers for diabetes were MRSA of hospital origin. CA-MRSA emerges in the community without hospitalization as a risk factor. It spreads due to close contact in sport settings, schools, day care centers, military settings and prisons. The livestock associated MRSA colonizes different food animal species and may cause infections in humans. It is transmitted to human through direct contact with animals, environmental contamination, as well as eating or handling contaminated food.
With unidentified reasons, most community-acquired methicillin resistant S. aureus bacteria are not as resistant to antibiotics as that of the hospital-acquired MRSA strains and/or health-care associated MRSA. Many of CA-MRSA strains have so far retained susceptibility to a number of non-beta-lactam antimicrobials. However, most infections caused by health-care associated and/or hospital associated MRSA are difficult to treat as they are resistant to non-beta-lactam antibiotics in addition (WHO., 2014). In the case of LA- MRSA, although most of them are multi resistant to several antibiotics there are still sufficient treatment options against LA- MRSA infection. The methicillin resistance of S. aureus is developed by acquisition of chromosomal cassette mec element. The cassette consists gene called mecA which encodes the novel penicillin binding protein PBP2a.
β-lactam antibiotics contradict cell wall synthesis by inhibiting trans peptidase hence affect bacterial growth. In addition, β-lactam can activate autolysin, which degrades peptidoglycan. PBP2a is in contrast the substitution of PBPs with low affinity for all β-lactams. It therefore, associates with the cell wall synthesis despite the presence of methicillin type drugs including methicillin.
The mec gene cassette also carries several virulence factors due to the reason that virulence factors and antibiotic resistance are closely linked. The Antibiotic resistance genes and virulence genes in staphylococci including MRSA are hence often acquired by a single horizontal gene transfer involving the SCC, with other several genes coding various virulence factors. This assures that methicillin resistance of S. aureus strains is virulent than other S. aureus.
The macrolide-lincosamide-streptogramin (MLS) group of antibiotics especially erythromycin and its derivatives, Vancomycin, have been extensively employed for the treatment of methicillin-resistant Staphylococcus aureus (MRSA). However, as the result of antibiotic resistance problems, several alternatives to antibiotics including bacteriocins and bacteriophages are recently becoming in the spotlight for the treatments of drug-esistant Staphylococcus aureus infections including MRSA. Bacteriocins, are small ribosomally synthesized antimicrobial peptide (AMP) produced by bacteria and are lethal to other bacteria as a mechanism of defence. The peptides insert into the membrane of the target bacteria, causing pores on the membrane and consequently lysis is the general mode of action. Bacteriophages, are viruses that infect bacterial cells. They may be used as therapeutic agent to treat infections caused by multi-drug resistance bacteria and to alleviate the risk of transmitting pathogenic bacteria via food commodities.
Characteristics of Phage’s
Bacteriophages also known as phage’s are obligate intracellular parasite bacterial viruses which attach on the host cell, inoculate their genome into the cell and hijack the cellular machinery. They are composed of nucleic acid genome enclosed within a protein or lipoprotein coat. They attack bacteria, multiply within them, and then destroy them. They are “programmed” to destroy one or a few kinds of different strains of bacteria.
Phage’s exist in many shapes and sizes. Structurally, most of them consist of three parts: head, tail and tail fiber. The head, or capsid, may be round, oval or polyhedral and is composed of protein. It encloses nucleic acid which can be either DNA or RNA but not both. The tail is like a hollow tube through which nucleic acid passes in to the host cytosol during infection and tail fibers help bacteriophages to attach on the bacterial surface. The extreme end of the tail has the ability to become attached to specific receptor sites on the surface of phage-sensitive bacteria. Once the tail of the virus attaches itself to a cell, it literally digests its way through the wall of the host cell. The size of most bacteriophages in general ranges from 22-200 nm in length.
The most important feature of phage’s is their narrow host range i.e. they kill only specific bacterial strain and that makes them as potential antimicrobial agents. This feature of bacteriophages is very advantageous because unlike broad-spectrum antibiotics, phage can kill specific pathogenic bacteria without affecting the balance of beneficial bacterial microflora.
The bacteriophages undergo two types of life cycle: lytic cycle (virulen) and lysogenic cycle (temperate). In lytic cycle, bacteriophage attach on bacteria through various cell surface receptors such as lipopolysaccharides (LPS), teichoic acids and various structural proteins (OmpA, C and F) on bacterial cell wall and injects its nucleic acid into the cytosol. Soon after, phage nucleic acid hijack the whole bacterial synthetic machinery that direct synthesis of phage specific mRNA, structural proteins and various other components. Later on, different phage components are assembled to form mature viral particles that come out from cell by lysis of the cell wall. Approximately, a total of about 1000 viral particles may be released per cycle. In lysogenic cycle, phage nucleic acids get integrate in to the bacterial genome and replicate along with genome and passed on to the daughter cells. The integrated viral genome is called ‘prophase’ and this process is known as lysogeny. Under certain condition like high radiation, change in metabolism, stresses, phage genome gets excised and becomes a lytic phage (Figure 1). The primary step in investigating the potential phage or its products against MRSA for phage therapy involves the isolation and selection of a lytic bacteriophage.
Phage therapy as Alternative for Drug Resistant Bacterial Infections
Historically, researchers sometimes took back us to the evidences in Bible to relate the documentations of bacteriophage therapy as ancient as Book of Kings during which prophet Elisha cured general Naaman’s disease by commanding him to bathe seven times in the river Jordan. However, in modern science, the brief history of phage began in 1896 during which Ernest Hankin reported the antibacterial activity of the Ganges and Jumna rivers of India. Hankin suggested that unidentified substances in the rivers were responsible for destruction and limitation of spread of Vibrio cholera. Similar evidences supporting the previous works had been obtained by various researchers. However, no one have proposed virus for such antibacterial activity. In 1914, however, the British bacteriologist, Frederick Twort incriminated bacterial virus for the phenomena. The research conducted in 1915 by a French-Canadian microbiologist, Felix d’Herelle, revealed round zones without growth of bacteria on agar plate spread with membrane filtered emulsions of faeces of sick men and called the bacterial devoid area “plaques”. He consequently asserted that the plaques were caused by bacteriolytic substances which were later named “bacteriophage” (meaning bacterial eater) in 1917 and candidate it in 1920 as therapy against bacterial infections.
The extensive work on phage therapy to treat infections caused by Streptococcus and Bacilli had been carried out between 1920 to 1930 in USA. At the time “Staphylogel” and “gel labelled” bacteriophage preparation was manufactured by Eli Lilly Company. As the result, many papers that showed benefits of bacteriophage therapy in animal models had been published between 1950 and 1980. However, at the same time, antibiotics were discovered and widely used as the consequence of which bacteriophages had been rejected to be used as therapeutic agent in many countries including Europe and USA. Later bacteriophage therapy had been rediscovered by Smith and Huggins in 1980s as alternative to antibiotics resistance in bacteria. Since then, treatment of infections by phage was re-emerged and widely used in several countries including Poland, United States, Georgia and Russia. Currently, in many countries like France, Georgia, United States and others, there are several phage therapy centers working, and dealing with various human diseases.
Although, it is estimated that there are more than 1031 bacteriophages contained in the earth biosphere, only limited number of bacteriophages have been identified worldwide so far. Bacteriophages that infect various host bacteria have been isolated from diverse sources such as human faeces, animal faeces, food, water, soil and sewage.
Practically, phage therapy has been widely used to treat severe infections caused by multi-drug resistant pathogenic bacteria in human, animals and plants and it is now also employ to enhance the shelf life of meats, vegetables, fruits and stored plant parts. Antibiotic resistance is driving more Western patients towards phage-therapy clinics in Europe and America. The US National Institute of Allergy and Infectious Diseases now list phages as a research priority to fight the emergence of antibiotic resistant bacteria. Thus there is increasing demand for isolation and characterization of more bacteriophage against major clinical threats.
Phage Therapy to MRSA Infections
Although most of S. aureus phages known so far are temperate phages of Siphoviridae family, strictly lytic staphylococcal phages in Podoviridae and Myoviridae families have been used for therapy.
Various types of phage preparations have been used for treatments of Staphylococcal infections in animal and human. In the United States, for example, Staphylococcal phage lysate (SPL) which was previously used for animal protection have been licensed to be used for human usage. The Hirszfeld Institute of Immunology and Experimental Therapy of Poland has been producing staphylococcal phage’s for therapeutic purposes since the 1970s and currently advanced to produce nine monovalent staphylococcal phages’. Most of the phage’s are used under therapeutic experiments for treatment of patients with chronic bacterial infections resistant to antibiotic therapy and gave encouraging results of good responses.
Advantages of Phage Therapy Over Chemotherapy
Bacteriophages are the potential unique therapeutic agents that have many advantages over classical antibiotics. First, bacteriophages are very specific that target specific bacterial strains without affecting the normal beneficial bacterial micro flora of the body; whereas, most of the antibiotics are broad spectrum to kill specific pathogenic bacteria; and also the normal beneficial micro- flora of the body. Thus antibiotics disturb microbial balance and may cause severe side effects. Second, it is easy to get new bacteriophage against bacterial strain which had already developed resistant to a specific bacteriophage. However, developing new antibiotic against drug resistant bacteria is more difficult and complex. Third many antibiotics are allergens to patients. In such cases the best treatment for infections is phage therapy. Fourth, phages are easily manufactured and cost effective than antibiotics of which processes are costly, complex and time consuming. Bacteriphages have unique ability to multiply and increase their number at the site of infection thus treatment can be performed with very small dose. Antibiotics do not accumulate at the site of infection; they are normally metabolized in the body and removed from the body through urine. In addition, treating infections caused by multidrug resistant bacteria is often difficult while is easily treated by phage therapy. Moreover, since bacteriophages are nontoxic to human, there have not been reports of severe side effects of the treatment. In case of using antibiotics, however, they may cause severe side effects including allergic reaction, intestinal disorders and several secondary infections.
Moreover, for example in the treatment of MRSA infections, vancomycin, teicoplanin, chinupristin/dalfopristin and linezolide are the strongest and expensive antibiotics used. These drugs are administered only intravenously during the patient’s hospital stay, and this significantly augments the total cost of treatment. Only linezolide has a formulation that enables oral administration in outpatients. However, the cost of this therapy is over 177.9 U.S. dollars, which is higher than the total mean cost of a 6.5-week phage therapy (including the costs of medical service and diagnostic tests). Phage preparations can be administered to the patients orally and/or locally. This enables us to conduct phage therapy in outpatients, which is very important in chronic infections. Patients with chronic infections often need prolonged administration of antibiotics, which may significantly augment total costs. Phage therapy, due to its chronic character and the good tolerance of phages even in the form of lysates, can be particularly recommended for these patients.
Isolation and Identification Methods of Phages
Phages are ubiquitous and can be easily isolated from their natural sources such as marine water, soil, sewage, wastes, fermented product and food including unprocessed vegetables and human and animal bodies. The isolated phages are purified by using filtration, ultracentrifugation, polyethylene glycol method and various forms of chromatography. The purification methods normally remove ruptured bacterial ghost cells, uninfected cells and other components of bacteria. In general terms, all most all bacteria possess their own bacteriophages that can be isolated from the environment where the host bacteria dowel.
The samples suspected to hold the phage are used as material to look for the phage in. These includes, mouth wash, milk, waste water etc. MRSA isolates are used as host for lytic phage isolation against MRSA. Briefly, Brain Heart Infusion (BHI) broth and bacterial culture obtained by adding BHI broth onto a 24 h fresh slant agar culture of S. aureus is added into the bottles containing the sample to be tested for the presence of specific phage and the bottles are incubated at 37°C for 24 h in shaker incubator. After the incubation, suspensions are centrifuged and the supernatants are filtered through a 0.22 μm filter. To determine the phage-host sensitivity, bacterial eluate is prepared by adding BHI broth onto the fresh S. aureus slant culture and vortexed vigorously. The eluate is added onto soft agar and plated on BHI agar plates. The suspected phage suspension is dropped on the agar plates containing host bacteria, and the plates are incubated aerobically at 37°C for 24 h. Bacteriophage activity is evaluated by the presence of plaque formation on BHI agar plates.
Several phage preparations have been developed by various commercial companies. Eli Lilly Company has been known to produce phages against Syaphylococci, Streptococci, E. coli and various other infectious pathogenic bacteria for the human use. Important examples of commercially obtainable bacteriophage preparations by the company include Ento-lysate, Staphylo-lysate, Neiso-lysate and Colo-lysate. In addition, D'Herelle's commercial laboratory in Paris produced at least five phage preparations against various bacterial infections. These preparations include Bactécoli-phage, Bacté-rhino-phage, Bacté-intesti-phage, Bacté-pyophage, and Bacté-staphy-phage. These preparations of the aforementioned companies mainly contain phage-lysed, sterile cultures of the host bacteria (e.g., Cololysate, Ento-lysate, Neiso-lysate, and Staphylo-lysate) or the same preparations in a water-soluble jelly base (e.g., Colojel, Ento-jel, and Staphylo-jel). Diabetic wounds, chronic infections, abscesses, suppurating wounds, vaginitis, acute and chronic infections of the upper respiratory tracts, and mastoid infections have been treated using these preparations.
Mode of delivery of bacteriophages
The potential and the most frequently reported route of administration of phages include topical, oral, rectal, and parenteral; topical administration to chronic wound infections. In such infections, phage cocktails combinations of a variety of phages have been used. One product used at the Eliava Institute is Phage BioDerm, a biodegradable polymer wound dressing impregnated with ciprofloxacin, benzocaine, chymotrypsin, bicarbonate, and 6 lytic phages (Pyophage) with activity against Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Streptococcus species, and Proteus species. Other potential means of topical administration include sprays, aerosols, lozenges, mouthwash, suppositories, bandages, eye drops, and tampons. Intra-pleural administration and bladder irrigation are also feasible.
Gastrointestinal infection and systemic infections are successfully treated with oral delivery of phage (Stanford et al., 2010). The main difficulty with phage delivery through oral route is that phage can be inactivated in the highly acidic condition of the stomach. In order to avoid such problem, polymer microencapsulated phages are used to protect phage from inactivation by acid and also enhance efficacy of phages. Other way of neutralizing acidity of stomach is use of sodium bicarbonate or sodium bicarbonate mineral water before administration of phages.
Local administration is the most successful route of phage administration where phage suspensions are directly applied on the infected area. In addition, phage can also be administered to human by intravenously (IV), intaperitonial (IP), intramuscular (IM), and subcutaneous (SC) methods.
Pharmacology and pharmacokinetics of phage therapy
In case of chemical drugs, the effect of drug on the body as well as the body’s impact on drugs is known as pharmacology. Pharmacokinetics is explanation of a drug’s potential to arrive in the near of specific target cells or tissues which are enough to achieve primary pharmacodynamic effect. This explanation is to differentiate in to distribution, absorption, metabolism as well as elimination of drugs. In case of bacteriophage, metabolism represents inactivation of phage because host immune system interacts and inactivates the phage or activation due to phage replication inside the host body. For success of a phage therapy, generation of adequate number of phages is necessary in the immediate vicinity of specific target bacteria that cause pathogen eradication from the body at some adequate rate. Bacteriophage will increase their sufficient number through in situ replication in host body and so- called active treatment. Thus the main goal pharmacologically is to gain sufficient densities of phages in the vicinity of target bacteria that lead to bacterial eradication.
Major Obstacles of Phage Therapy
Despite efforts have been done, bacteriophage therapy remains an underutilized option in Western medicine due to challenges such as regulation, limited host range, bacterial resistance to phage’s, manufacturing, side effects of bacterial lysis, and delivery. Recent advances in biotechnology, bacterial diagnostics, macromolecule delivery, and synthetic biology may help to overcome these technical hurdles. These research efforts must be coupled with practical and rigorous approaches at academic, commercial, and regulatory levels in order to successfully advance bacteriophage therapy into clinical settings.
Although it seems everything in favour of using lytic phage’s as therapeutics but they are not commonly used to the extent of expectations and sometimes their efficacy is a matter of controversy. One of the most significant factors associated with the efficacy of phage therapy is the paucity of an appropriate placebo-controlled studies. Some obstacles in the application of phage therapy are: heterogeneity and ecology of both phage’s and bacteria, selection of highly virulent phage’s against targeted host bacteria, mono-phage preparations against different bacteria, selective phage cocktail appropriately characterize or titre phage preparations, lateral gene transfer of virulence factors and antibiotic resistance, restriction modification degradation of phage DNA upon infection and resistance mutations in bacterial genes for adsorption, lysogeny and lysogenic conversion. Moreover, common problems confronting phage therapy include phage-host interactions, phage characterization to determine the virulence of the virus against the target, necessity of neutralizing gastric pH prior to oral administration, immunogenicity to provoke antibody production against the phage, and phage gene recombination in human genes. However, the major drawbacks of phage therapy are discussed as follows.
The primary obstacle of phage therapy is the quick removal of bacteriophages by liver, spleen and other different filtering organs of reticuloendothelial system. This action can lower the densities of phage’s to a level it cannot attack the pathogenic bacteria of interest so that the phage’s are not sufficient to combat against the infection.
Other major obstacle for phage therapy is that when the phage’s administered intravenously, humoral immune system is activated to result in production of antibodies. These phage specific antibodies rapidly inactivate the phage’s. However, antibodies are not produced, if local and oral route of administration used. The issue of bacteriophage interactions with the mammalian immune system and its components is still not precisely defined. Evidence increasingly suggests that phage’s influence mammalian immune responses, including the attenuation of specific and nonspecific immune reactions, and maintenance of local immune tolerance to gut microorganism-derived antigens. Although innate immunity cannot be entirely separated from its adaptive counterpart, for the purpose of clarity, we will also discuss antiphase immunity according to the mechanism(s) of neutralization and clearance.
Modern Techniques of Improving Phage Therapy
Therapeutic and prophylactic application of phages are encouraging over conventional medicine, there is an obvious need to develop phage therapy in wide range of applications. Though the first reported discovery of antibacterial-like activity linked to bacteriophages was made in 1896, where Hankin stated the waters of the Ganga and Jamuna rivers might have restricted outbreaks of cholera. Since then due to lack of information regarding basic phage biology and early antibacterial drugs together restricted the development of phage therapy. In recent years due to increased threat of bacterial resistance to drugs and lack of new antibacterial drug development, opened new opportunities to develop phase therapy. Moreover, with increased information regarding phage biology and improvements in molecular biology researchers have now progressed to the point where sufficient knowledge acquired to improve phase therapy as next-generation application.
Genetic engineering helps to improve the phage therapy to overcome many obstacles involved in using wild type phages. For instance, while treating gram-negative bacteria cell wall liberates endotoxin which is harmful and cause bacterimia, septicemia etc. To overcome this difficulty, several bacteriophages have now been developed through genetic engineering that are noneplicating and non-lytic. These genetic engineered bacteriophage, encode specific proteins that are deleterious to bacteria with release of only small amount of endotoxin. Engineered bacteriophage can enhance the killing of antibiotic-resistant bacteria, persistent cells, and biofilm cells, reduce the number of antibiotic-resistant bacteria that arise from an antibiotic-treated population, and act as a strong adjuvant for other bactericidal antibiotics. Cock tail of phages that are genetically modified would be more helpful in addressing a wide variety of bacterial infections that are otherwise resistant to the latest generations of antibiotics. Likewise, many researchers are using genetic engineering approach to advance the phase therapy.
There are many genes in bacterial genome whose products are harmful to human on which antibiotics cannot target directly. Therefore, genetic engineered bacteriophages were developed that have ability to overexpress proteins to target the bacterial gene networks on which the antibiotics cannot works and increases destruction of bacteria by antibiotics. With recent advances of DNA synthesis, synthetic phages were developed and well-suited for incorporating targets, identified via systems biology, in a modular fashion to create effective antibiotic adjuvants. These ever improving technologies will enable large-scale modifications of phage libraries to produce antibiotic-adjuvant phage that target different gene networks and that can be applied with different antibiotic drugs against a wide range of bacteria. Combination therapy which couple’s antibiotics with engineered antibiotic adjuvant phage is a promising antimicrobial strategy for the future.
Many bacteriophages possess lytic enzymes such as holins and lysins that have ability to battle against host to survive. They have yet to be exploited. Now new techniques are employed in which bacteriophages are used in combination with phage lytic enzymes that enhance the killing capacity of bacteriophages. All of these enzymes are highly evolved molecules designed for a specific purpose, to quickly destroy the bacterial cell wall, and as such, nanogram quantities of enzyme are sufficient to sterilize a 107 bacterial suspension in seconds. Because of the serious problems of resistant bacteria in hospitals, day care centers, and nursing homes, particularly Staphylococci and Pneumococcal, such enzymes may be of immediate benefit in these environments. Thus, we may add phage enzymes to our armamentarium against pathogenic bacteria.
Conclusion
From the above discussions one can conclude that bacterial drug resistance is becoming great bottleneck of public and veterinary medicine in the world. The problem is serious particularly in bacteria like MRSA because they are prone to resist vancomycin which is drug of choice. More over MRSA can live on the body or cause disease in human from simple to life threatening and even to death. To combat such problem, the best method now day available is phage therapy.
Phage therapy has many advantages over chemical drugs that first it attacks only specific groups (species or strain) of bacteria so that it rescues the normal flora of the body. Second, it is less immunogenic and non toxogenic. In addition, its preparation is easy and cheaper than chemical drugs. Moreover, developing drug against drug resistant bacteria is difficult. However, finding effective phage against phage-resistant bacteria is easy and needs short time.
Moreover, the obstacles encountering phage therapy include limited host range, bacterial resistance to phages, manufacturing, difficulty of finding lytic phages, and rapid clearance of the phage from the body. Even studies conducted so far have still tided to trial and did not pass to clinical application.
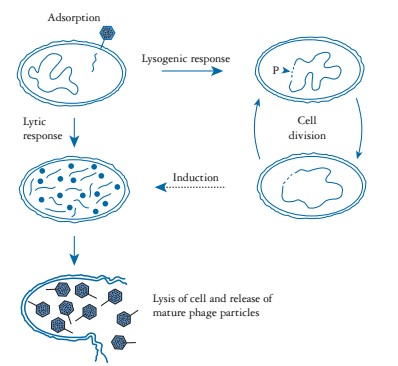
Figure 1: Life cycle of bacteriophage. (Adopted from: Nicholl (2008).
Citation: Feyissa N, Jirata D, Alemu T, Desalegn A (2019) Review on Phage Therapy as Alternative Treatment for Meticillin-Resistant S. Aureus (MRSA) Infections: Identification and Application of Phage. J Sports Sci Physical Ther: JSSPT-104.