Annals of Medical & Surgical Case Reports
Case Report
Scrotal Mass of Paratesticular Origin; Uncommon but Clinically Significant
Seneviratne B*
Department of Pathology, University of Sri Jayewardenepura, Sri Lanka
*Corresponding author: Bimalka Seneviratne, Department of Pathology, University of Sri Jayewardenepura, Sri Lank, Tel: +94 112 801 025; E-mail: bimalka03@yahoo.com
Citation: Seneviratne B (2019) Scrotal Mass of Paratesticular Origin; Uncommon but Clinically Significant. Ann Med & Surg Case Rep: AMSCR-1000024
Received date: 15 October, 2019; Accepted date: 20 October, 2019; Published date: 30 October,2019
Introduction
The majority of tumours within the scrotum are of testicular origin and only 2-3% arise in paratesticular tissue [1]. Paratesticular tissue comprises of different types of cells which could be of epithelial, mesenchymal or mesothelial origin. Nevertheless, a wide range of tumours are known to develop in paratesticular tissue [2]. However, the majority of tumours in the paratesticular region are benign, and some of the common entities include lipoma, adenomatoid tumour and leiomyoma. Less than one third of tumours are malignant [3,4]. In view of the diversity of paratesticular tumours it is important to arrive at a definitive diagnosis by histological evaluation [5] of the resected specimen. Due to the infrequent occurrence of these cases and limited data on prognostic implications, there is significant variation in the management of patients about neoadjuvant therapy and follow- up [6]. A consensus opinion on best modalities of treatment needs to be reached for the future management of patients with paratesticular tumours [7].
Keywords: Liposarcoma; Paratesticular sarcoma; Scrotal mass
Case report
Eighty-five years old male presented to the surgical clinic with progressively enlarging scrotal mass over the last 3 months. He complained of a dull pain in the scrotal and inguinal region not related to any posture. Ultrasonography confirmed the presence of a large necrotic mass adjacent to the left testis. There was no radiological or clinical evidence of lymphadenopathy. In view of the highly suspicious imaging results, patient underwent a left sided radical orchidectomy with high spermatic cord ligation.
Pathological findings
Gross examination revealed a large, lobulated mass measuring 19 × 14 × 12 cm. Cut surface was partly yellow in colour and revealed lipoma-like areas (Figure 1). However, the bulk of the tumour consisted of a fleshy, white mass with necrotic and hemorrhagic areas (Figure 2). Left testis which was identified adjacent to the mass measured 3.5 × 2 × 2 cm and was macroscopically normal. Microscopic examination of the tumour revealed cellular areas composed of spindle shaped cells arranged in short fascicles (Figure 3). There was nuclear atypia and increased mitotic activity (? 10 / 10 hpf). In addition, there were multinucleated cells and bizarre forms. Spindle cell areas were admixed with a well-differentiated, spindle cell liposarcoma component having occasional lipoblasts (Figure 4). Tumour showed large areas of necrosis. Testicular tissue was uninvolved by tumour and consisted of atrophic seminiferous tubules with no evidence of spermatogenesis.
Discussion
Morphological findings were suggestive of a paratesticular sarcoma. The presence of de-differentiated spindle cell areas and well-differentiated, spindle cell liposarcoma component with abrupt transition, and the occurrence of lipoblasts raised the possibility of de-differentiated liposarcoma [8,9]. Immunohistochemistry results complemented the morphological findings by identifying the lipoblasts with the S-100 immuno histochemical stain (Figure 5). Negative staining for immune histochemical markers desmin, myogenin and smooth muscle actin enabled to rule out leiomyosarcoma and rhabdomyosarcoma (Figure 6). Fibrosarcoma which comes in the differential diagnosis was eliminated following the identification of lipoblasts [10]. Undifferentiated pleomorphic sarcoma which is a diagnosis of exclusion was not considered because of the positive staining for S-100. Poorly differentiated carcinoma was ruled out in view of the negative staining for cytokeratin (AEI / AE3), and the presence of well-differentiated, spindle cell liposarcoma component.
Conclusion
Primary tumours of the paratesticular region are uncommon. However, in the elderly a significant proportion constitute of paratesticular sarcomas. The majority of paratesticular sarcomas arise from the spermatic cord. In most of the instances it is not possible to differentiate testicular and paratesticular tumours by clinical examination. Ultrasound has been a reliable investigation in the confirmation of the origin of scrotal masses with a sensitivity of over 95% [11]. Radiological results also provide valuable information about the nature of the tumour. High level of suspicion is required as the cornerstone of management for malignant paratesticular tumours is radical orchidectomy with high spermatic cord ligation. These tumours have an aggressive behavior and histological confirmation is essential to plan out the management. Immunohistochemistry to identify tumour specific biomarkers is essential as there could be overlapping features between different types of paratesticular sarcomas. Staging of paratesticular sarcomas is done after considering the MRI and CT scan findings [12]. Multidisciplinary approach would enable to discuss the aggressiveness and histological grade of the tumour and to select the most appropriate treatment option [13]. Although uncommon adherence to best practice guidelines would undoubtedly prove to have better results.
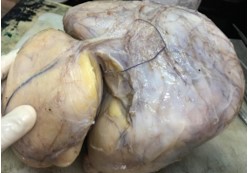
Figure 1: Large lobulated mass with a thin pseudocapsule.
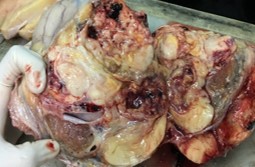
Figure 2: Fleshy white tumour with necrotic & hemorrhagic areas (broad arrow). Left testis adjacent to the tumour (thin arrow).
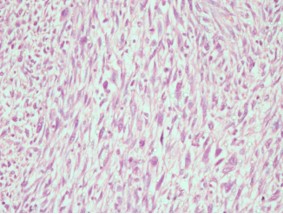
Figure 3: Spindle cell areas (de-differentiated liposarcoma) H & E stain × 40.
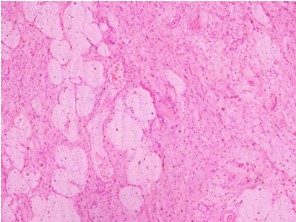
Figure 4: Well-differentiated, spindle cell liposarcoma component (H & E stain × 10).
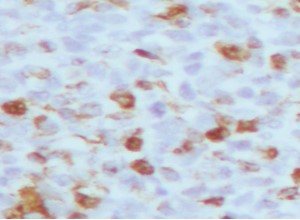
Figure 5: S 100 ( × 40)- scattered lipoblasts showing positive staining.
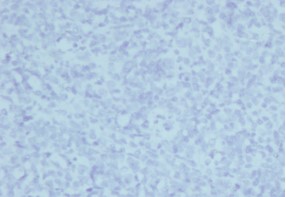
Figure 6: Desmin (× 10) - negative staining of the de-differentiated spindle cell area.
Citation: Seneviratne B (2019) Scrotal Mass of Paratesticular Origin; Uncommon but Clinically Significant. Ann Med & Surg Case Rep: AMSCR-1000024.